BETTER EVIDENCE TO BUILD CONFIDENCE
Abbott provides a robust body of clinical evidence encompassing clinical data and outcomes from tens of thousands of patients across our structural heart portfolio of products.*
IN-DEPTH DATA FOR STRUCTURAL HEART PRODUCTS
With our Clinical Insights and Bulletins, you can take a deeper dive into clinical outcomes related to structural heart products. Designed for ease of viewing, these pieces give you a broader perspective about study outcomes along with product safety and performance.
Transcatheter Edge-to-Edge Repair (TEER)
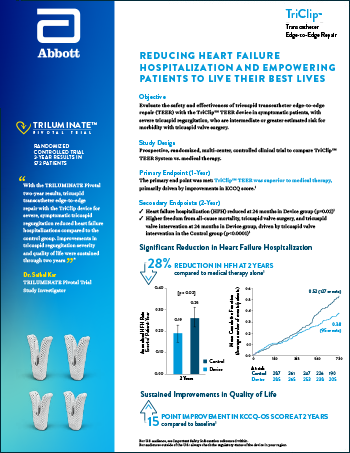
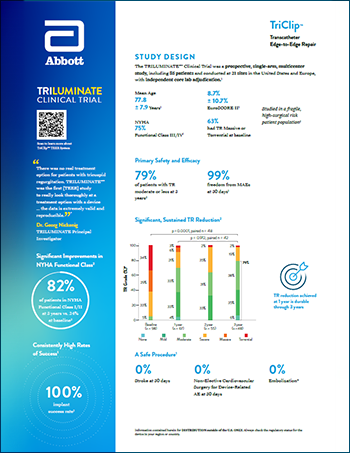
TriClip™ for TEER
Read moreReducing heart failure hospitalization and empowering patients to live their best lives
Transcatheter Aortic Valve Implantation (TAVI)

Navitor™ TAVI System: 5-year durability
Read moreDevice design and favorable 5-year durability reinforce Navitor™ TAVI
long-term outlook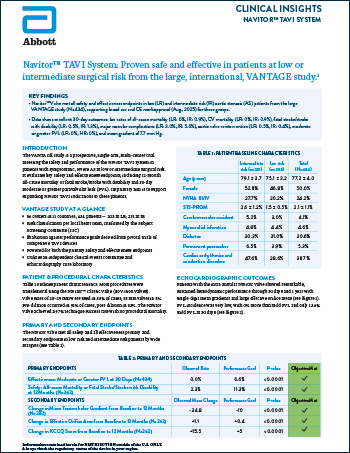
Navitor™ TAVI System: VANTAGE Study
Read moreProven safe and effective in patients at low or intermediate surgical risk from the large, international, VANTAGE study.

Navitor™ TAVI System: 10.6% New PPI with Experience
Read more10.6% New PPI With Navitor™ Vision Valve After 20 Procedures
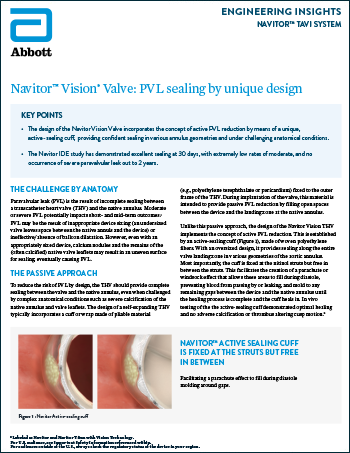
Navitor™ Vision Valve: PVL Sealing by Unique Design
Read more0% Moderate to Severe PVL With Navitor™ Active-Sealing Cuff

Navitor™ Valve: Small Aortic Annuli
Read moreSingle-Digit Mean Gradients With The Self-Expanding Navitor™ Valve

Navitor™ TAVI System: Minimizing PPI Risks
Read morePPI Rates of 10% or Lower Using Best Implant Practices
Structural Interventions

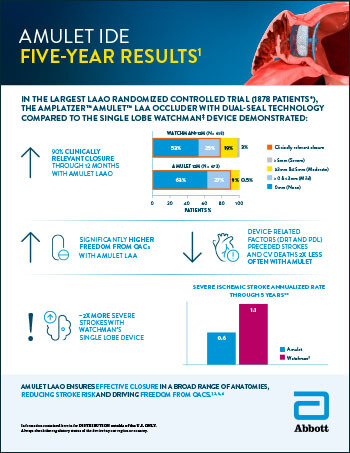
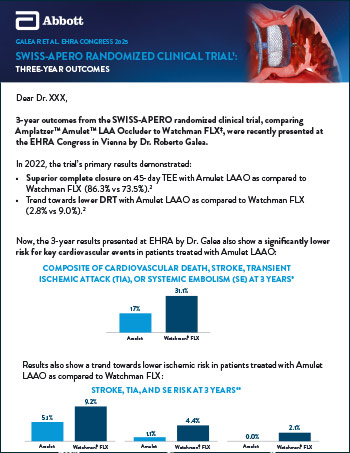
Amplatzer™ Amulet™ LAA Occluder
Read moreEmerge LAA Post-Approval Study: Presentations and Publications Year-in-Review

Amplatzer™ Amulet™ LAA Occluder
Read moreLAAO Stroke Risk Reduction: Device-Related Factors Considerations
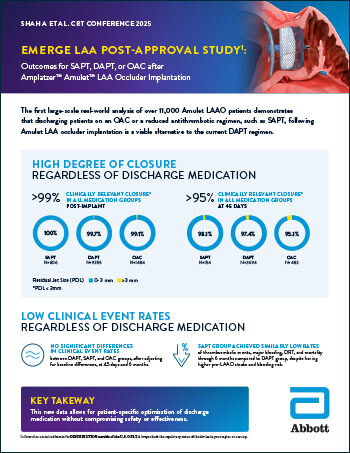
Amplatzer™ Amulet™ LAA Occluder
Read moreEmerge LAA Post-Approval Study: Outcomes for SAPT, DAPT, or OAC after Amplatzer™ Amulet™ LAA Occluder Implantation
Surgical Valve Solutions

Epic™ Mitral Stented Tissue Valve with Linx™ AC Technology
Read more
Epic Mitral – Ten Year Clinical Outcomes of SMVR in a Medicare Population1
Epic™ Supra Aortic Stented Tissue Valve with Linx™ AC Technology
Read moreTen-Years Outcomes of a Contemporary Supra-Annular Porcine Aortic Bioprosthesis in a Medicare Population1

Epic™ Mitral and Epic™ Supra Stented Tissue Valves
Read more
Three-Year Outcomes of Valve-in-Valve Intervention within the Epic™ Supra and Epic™ Mitral Valves in a Medicare Population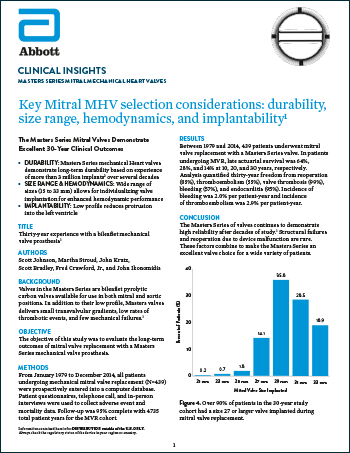
Masters Series Mitral Mechanical Heart Valves
Read moreKey Mitral MHV Selection Considerations: Durability, Size Range, Hemodynamics and Implantability1
Tissue Heart Valves
Read moreComparison of Failure Modes of Bioprosthetic Porcine Valves versus Bovine Pericardial Valves
Mechanical Heart Valves
Read moreBioprosthetic vs. Mechanical Aortic Valve Replacement in Patients 40–75 Years
CLINICAL DATA | TRANSCATHETER VALVE SOLUTIONS
CLINICAL DATA | STRUCTURAL INTERVENTIONS
CLINICAL DATA | SURGICAL VALVE SOLUTIONS
MAT-2004712 v24.0 | Item approved for OUS use only.