TRICLIP™ TRANSCATHETER TRICUSPID VALVE REPAIR CLINICAL DATA
TriClip™ Transcatheter Edge-to-Edge repair offers a minimally invasive treatment option for patients with severe symptomatic tricuspid regurgitation (TR).
TRICLIP™ TRANSCATHETER TRICUSPID VALVE REPAIR CLINICAL DATA
TriClip™ Transcatheter Edge-to-Edge repair offers a minimally invasive treatment option for patients with severe symptomatic tricuspid regurgitation (TR).
THE STANDARD OF CARING FOR TR
TriClip TEER has shown meaningful outcomes in both real-world and randomized clinical data. The bRIGHT study and TRILUMINATE™ Pivotal Trial demonstrated the safety and effectiveness of TriClip TEER in the treatment of severe tricuspid regurgitation (TR).1,4
TRILUMINATE™ PIVOTAL TRIAL
Exceptional safety.1,2
Life-changing impact.1,4
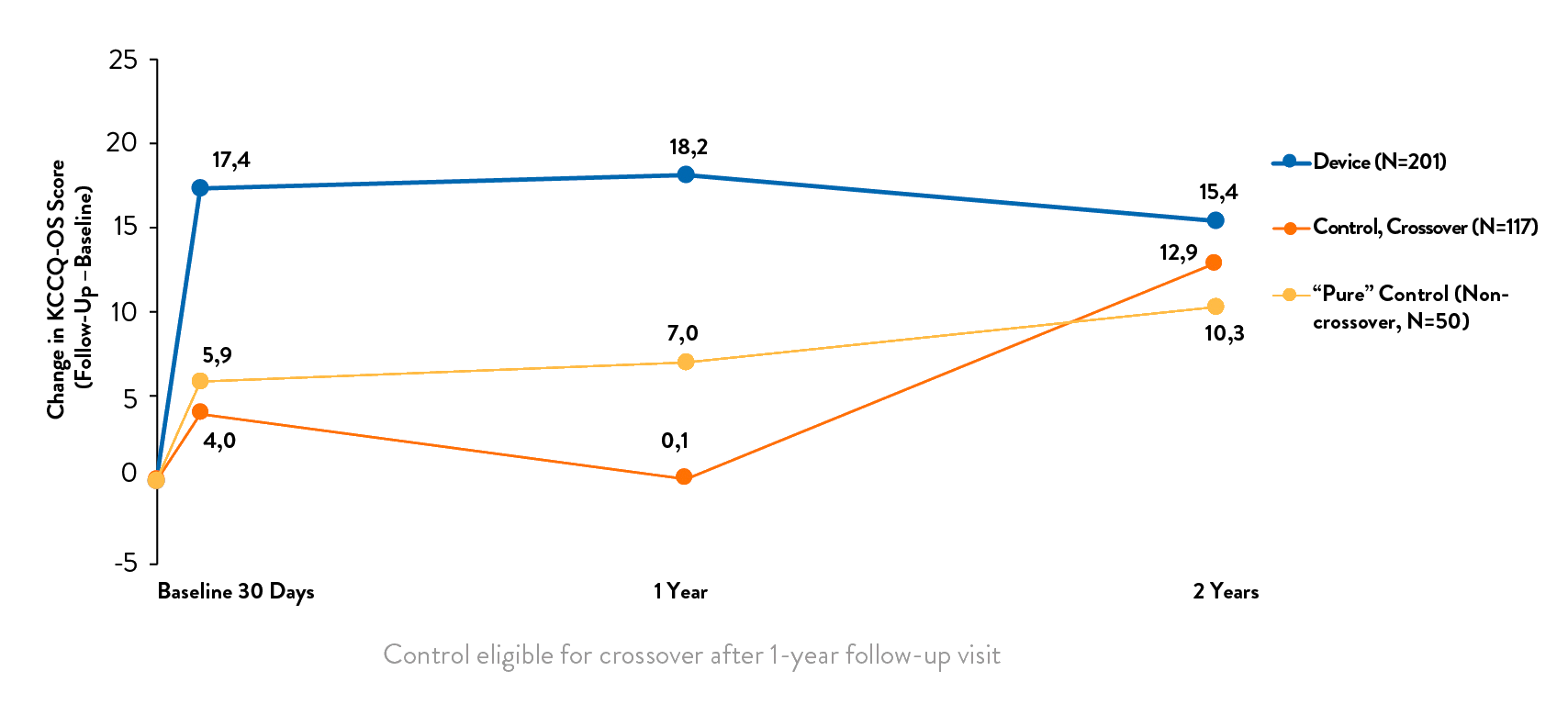
The TRILUMINATE™ Pivotal Trial demonstrated that TriClip TEER Therapy was superior to medical therapy alone reducing risk of heart failure hospitalization and improving quality of life.
Remarkable and sustained TR reduction3
TR grade (Core Lab)
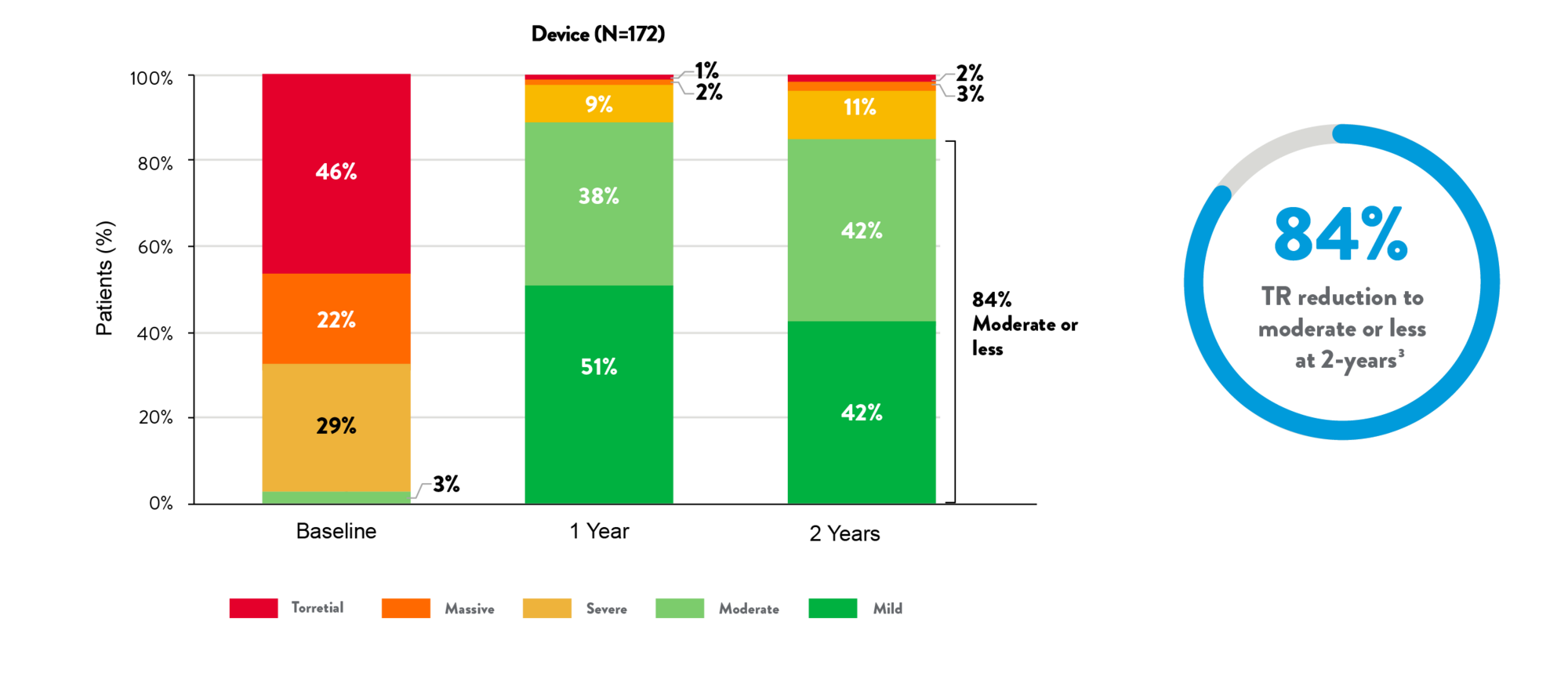
- Exceptional safety at 30 days1,3
98.9%
FREEDOM
FROM MAEs0.9%
NEW PACEMAKER
IMPLANTATION0%
DEVICE THROMBUS
99.6%
SURVIVAL
0%
NONELECTIVE CV
SURGERY FROM
DEVICE-RELATED AE0%
DEVICE EMBOLIZATION
- Significant improvement in health-related quality of life3
- Baseline population characteristics5
73%
OF PATIENTS HAD MASSIVE
OR TORRENTIAL TR4.3±0.8cm
TRICUSPID VALVE
ANNULUS DIAMETER38%
OF PATIENTS WITH PRIOR
MITRAL OR AORTIC VALVULAR INTERVENTION17%
OF PATIENTS HAD A CRT, CRT-D, ICD,
OR PERMANENT PACEMAKER
bRIGHT STUDY
Proven across a broad
range of anatomies4,6
The bRIGHT Study demonstrated that TriClip™ TEER significantly reduced TR across a broad range of anatomies - in a safe and effective procedure - resulting in durable and meaningful clinical outcomes.
Designed to maximize TR reduction2,4,7
TR grade (Core Lab)
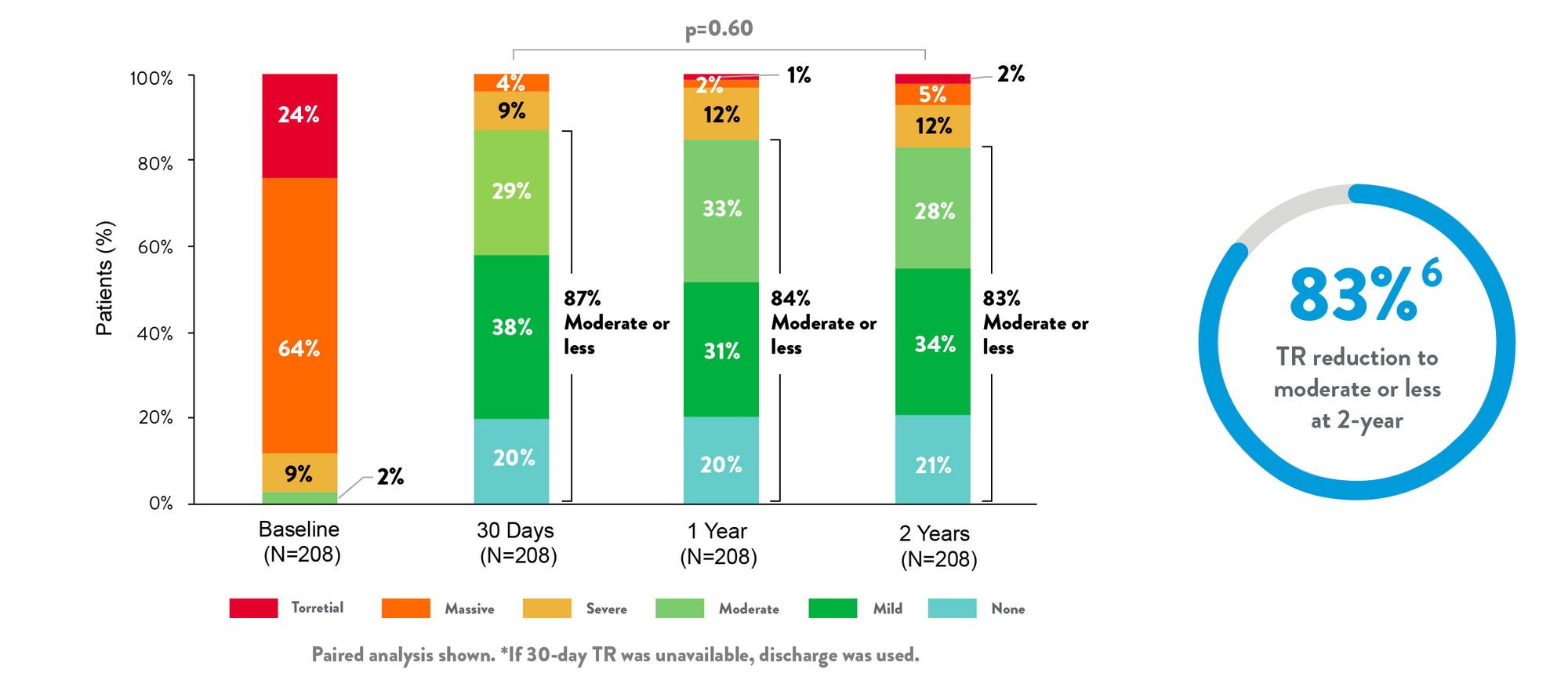
- Successfully treated a broad range of anatomies6
- Proven durability backed by clinical data
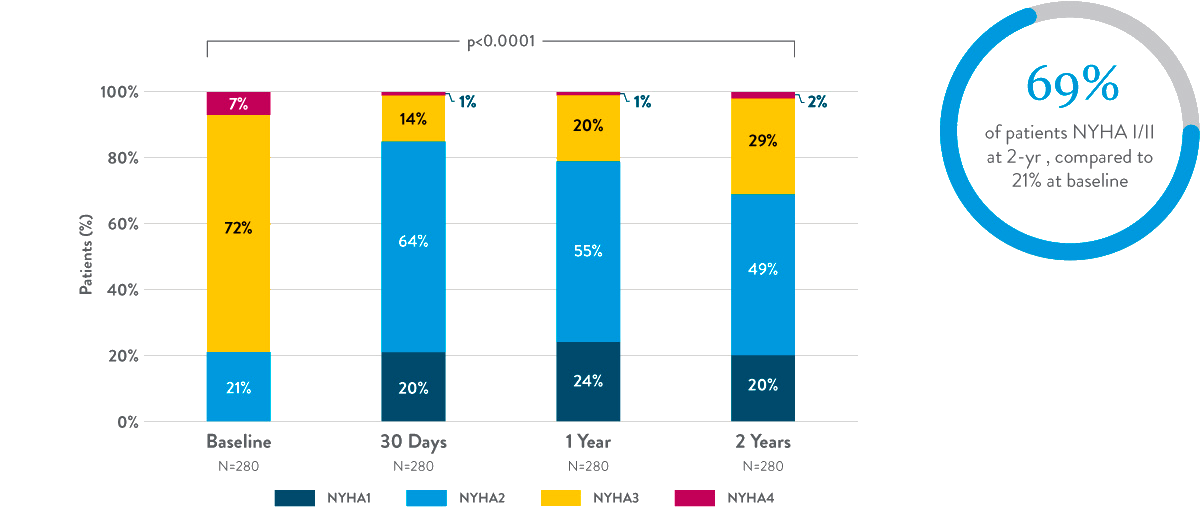
NYHA Functional Class7
KCCQ - OS Score2
- TriClip TEER demonstrated procedural success and an exceptional safety profile8
HIGH PROCEDURAL
SUCCESS99.0%
Implant
sucess rateSHORT
DEVICE TIME
76±39
Minutes
HIGH SAFETY
PROFILE
97.5%
Freedom
from MAEs
at 30 days
99.0% survival at 30 days0.2% TV reintervention
0.0% embolization
TRILUMINATE™ TRIAL
Proven safety and effectiveness
The TRILUMINATE™ Trial demonstrated that TriClip TEER safely and effectively reduces TR and HF hospitalizations.
93%
SURVIVAL
AT 1 YR10
100%
IMPLANT
SUCCESS10
91%
ACUTE PROCEDURAL
SUCCESS RATE10
0%
STROKE10
AT 30 DAYS
0%
CONVERSION TO
SURGERY10
AT 30 DAYS
- Significant and durable TR reduction11
Durable reduction in tricuspid regurgitation (TR)
- Significant improvement in functional status and quality of life11
Durable improvements in NYHA
≥10-point KCCQ-OS score improvement was observed in 50% of subjects at three years.11
MAT-2006556 v13.0 | Item approved for OUS use only.