Structural Interventions
The Amplatzer™ portfolio of structural intervention occluders provides minimally invasive treatment options for the closure of congenital heart defects and for stroke risk reduction. The procedures are performed through a minimally invasive, transcatheter approach, which prevents the need for open heart surgery and helps facilitate faster recovery and lower complication rates. The Amplatzer line has built a reputation for innovation by pioneering structural heart defect occluder devices and remains the leader in treatment worldwide.
Structural Interventions
The Amplatzer™ portfolio of structural intervention occluders provides minimally invasive treatment options for the closure of congenital heart defects and for stroke risk reduction. The procedures are performed through a minimally invasive, transcatheter approach, which prevents the need for open heart surgery and helps facilitate faster recovery and lower complication rates. The Amplatzer line has built a reputation for innovation by pioneering structural heart defect occluder devices and remains the leader in treatment worldwide.
STRUCTURAL INTERVENTIONS
BUILT FOR THE AGES
Thanks to the vision of our founder, Dr. Kurt Amplatz, and the bold, committed physicians who built upon his work, a device was pioneered that could close the holes in the hearts of the tiniest babies: the Amplatzer Piccolo™ Occluder. Watch how we carry on this exciting legacy today.

AMPLATZER™ AMULET™ LEFT ATRIAL APPENDAGE OCCLUDER
STROKE RISK REDUCTION
The Amplatzer Amulet LAA Occluder is designed to treat patients with atrial fibrillation who are at risk of ischemic stroke. The device offers immediate closure1of the left atrial appendage (LAA), reducing the risk of stroke and immediately eliminating the need for oral anticoagulants.2
AMPLATZER™ TALISMAN™ PFO OCCLUDER
STROKE RISK REDUCTION
The Amplatzer PFO Occluder set the standard, pioneering treatment with a device developed specifically for patent foramen ovale (PFO) closure to reduce the risk of recurrent ischemic stroke.3 The Amplatzer Talisman PFO Occluder is built off this proven technology, with key enhancements to make it our most advanced and easy-to-use PFO closure device yet. With over 180,000 devices implanted worldwide,2 we are the leader in PFO closure.


AMPLATZER™ TALISMAN™ PFO OCCLUDER
STROKE RISK REDUCTION
The Amplatzer PFO Occluder set the standard, pioneering treatment with a device developed specifically for patent foramen ovale (PFO) closure to reduce the risk of recurrent ischemic stroke.3 The Amplatzer Talisman PFO Occluder is built off this proven technology, with key enhancements to make it our most advanced and easy-to-use PFO closure device yet. With over 180,000 devices implanted worldwide,2 we are the leader in PFO closure.

AMPLATZER™ SEPTAL OCCLUDER
CONGENITAL DEFECTS
The Amplatzer Septal Occluder is the standard of care for minimally invasive atrial septal defect (ASD) closure, with a variety of shapes, sizes, and features that facilitate precise placement. It is the most studied ASD closure device available today, with over 20 years of demonstrated clinical experience.4,5
AMPLATZER™ DUCT OCCLUDERS
CONGENITAL DEFECTS
The Amplatzer family of duct occluders offers an extensive line of devices for the closure of patent ductus arteriosus (PDA). Amplatzer Duct Occluders are designed to occlude PDAs of various shapes and sizes to meet the needs of your patients.
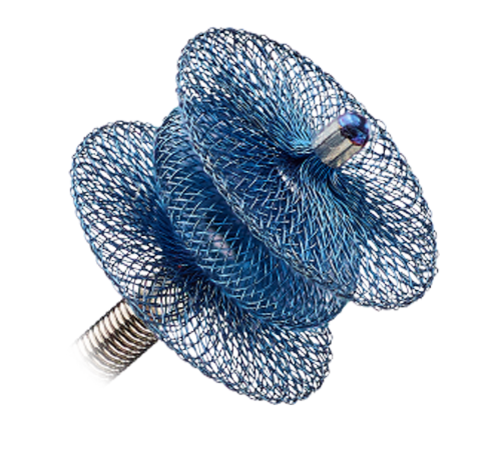

AMPLATZER™ DUCT OCCLUDERS
CONGENITAL DEFECTS
The Amplatzer family of duct occluders offers an extensive line of devices for the closure of patent ductus arteriosus (PDA). Amplatzer Duct Occluders are designed to occlude PDAs of various shapes and sizes to meet the needs of your patients.

AMPLATZER™ VSD OCCLUDERS
CONGENITAL DEFECTS
Ventricular septal defects (VSD) are the most common congenital heart defect,6 and one subtype of these defects is muscular VSD. The Amplatzer Muscular VSD Occluders are designed for complete closure of these type of defects.
AMPLATZER™ VALVULAR PLUG III
PARAVALVULAR LEAK
The Amplatzer™ Valvular Plug III is specially designed to provide effective closure for patients with a wide range of paravalvular leak morphologies. By providing an effective solution to this key issue, the Amplatzer Valvular Plug III improving life, quality and longevity to an increasing number of patients.7,8,9,10


AMPLATZER™ VALVULAR PLUG III
PARAVALVULAR LEAK
The Amplatzer™ Valvular Plug III is specially designed to provide effective closure for patients with a wide range of paravalvular leak morphologies. By providing an effective solution to this key issue, the Amplatzer Valvular Plug III improving life, quality and longevity to an increasing number of patients.7,8,9,10
MAT-2201554 v3.0 | Item approved for OUS use only.