AMPLATZER™ AMULET™ LAA OCCLUDER
Designed to treat patients with non-valvular atrial fibrillation (AFib) who are at risk of ischemic stroke, the Amplatzer Amulet LAA Occluder offers complete closure of the left atrial appendage (LAA) and immediately eliminates the need for oral anticoagulants.1
REDUCE THE RISK OF STROKE,
ELIMINATE ORAL ANTICOAGULANTS
CLOSURE. ONCE AND FOR ALL.2,3
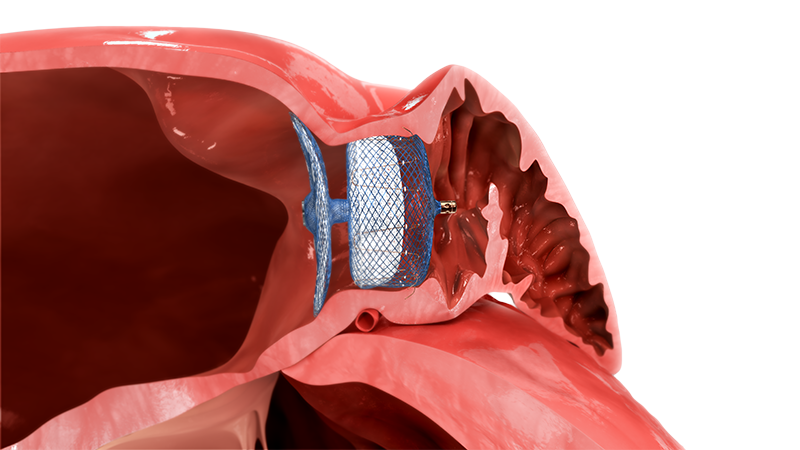
The Amplatzer Amulet LAA Occluder offers a dual seal design to treat virtually all morphologies and provide closure, once and for all.
For those patients who are either contraindicated for OACs or wish to discontinue use, LAAO with the Amulet Occluder is a viable option to reduce their risk of stroke.
Reducing Stroke Risk
Dr. Atman Shah, MD, Clinical Director, Cardiology, University of Chicago, United States
“I choose Amulet for a number of different reasons. What’s unique about the Amulet [device]…Its dual seal really offers patients the best way to have their appendage closed off and gives physicians confidence knowing that appendage will stay closed off.”
PATIENTS WHO BENEFIT
Life-long medication to reduce stroke risk is not an option for many patients. The Amplatzer Amulet LAA Occluder permanently closes this primary stroke pathway without requiring these patients to endure the challenges of Oral Anticoagulants (OACs).
Why Amplatzer Amulet? Meet Terry
The Amulet Occluder can be a good option1, 4-6 for patients who:
The long-term bleeding risks associated with OACs such as warfarin are particularly concerning for patients with active lifestyles or occupations. Consider Amplatzer Amulet Occluder for active patients.

Rita (AGE 65)
Rita has NVAF and a CHA2DS2-VASc score of 4. She is reducing her stroke risk without having to limit her travel and play with her grandchildren.
NVAF patients who want to remain active should know that there is an alternative.
A fall can erode a patient’s confidence and willingness to engage in everyday tasks. It can also lead to a major bleeding event. Consider Amplatzer Amulet Occluder when you or your patients are concerned about the risk of falls or bleeding.

Nathan (AGE 80)
Nathan, who has NVAF and a CHA2DS2-VASc score of 5, hit his head the last time he fell. He and his doctor want to reduce his stroke risk without increasing his risk for a major bleeding event.
NVAF patients at high risk for bleeding should know that there is an alternative.
OACs are incompatible with treatments for many comorbidities including depression or arthritis. Amplatzer Amulet Occluder is an alternative to oral anticoagulants that can increase the risk of drug interactions.

Michael (AGE 75)
Michael has Non-Valvular Atrial Fibrillation (NVAF) and a CHA2DS2-VASc score of 5. He is reducing his stroke risk without increasing his risk for drug interactions.
NVAF patients with comorbidities should know that there is an alternative.
Adverse effects, the cost of Novel Oral Anticoagulants (NOACs), or the monitoring required when taking warfarin, often make compliance a challenge for patients. Amplatzer Amulet Occluder is an alternative to life-long oral anticoagulants that can lead to noncompliance.

Sarah (AGE 73)
Sarah has NVAF and a CHA2DS2-VASc score of 4. She is reducing her stroke risk without having to take OACs for the rest of her life.
NVAF patients who struggle with compliance should know that there is an alternative.
 </picture>
</picture>
ARE CONTRAINDICATED
TO OACs
For patients with poorly controlled or uncontrolled hypertension, oral anticoagulants are contraindicated. Amplatzer Amulet Occluder is an alternative to life-long oral anticoagulants that are not a safe choice for these patients.

Regina (AGE 55)
Regina, who has NVAF and a CHA2DS2-VASc score is 4, has had poorly controlled hypertension for 10 years. Her doctor wants to reduce her stroke risk without jeopardizing her safety.
NVAF patients with poorly controlled hypertension should know that there is an alternative.
 </picture>
</picture>
HAVE HAD
A
BLEEDING EVENT
The long-term bleeding risks associated with OACs such as warfarin are particularly concerning for patients with active lifestyles or occupations. Consider Amplatzer Amulet Occluder for active patients.

Rita (AGE 65)
Rita has NVAF and a CHA2DS2-VASc score of 4. She is reducing her stroke risk without having to limit her travel and play with her grandchildren.
NVAF patients who want to remain active should know that there is an alternative.
 </picture>
</picture>
ARE AT
HIGH
RISK FOR FALLS
A fall can erode a patient’s confidence and willingness to engage in everyday tasks. It can also lead to a major bleeding event. Consider Amplatzer Amulet Occluder when you or your patients are concerned about the risk of falls or bleeding.

Nathan (AGE 80)
Nathan, who has NVAF and a CHA2DS2-VASc score of 5, hit his head the last time he fell. He and his doctor want to reduce his stroke risk without increasing his risk for a major bleeding event.
NVAF patients at high risk for bleeding should know that there is an alternative.
 </picture>
</picture>
ARE AT RISK
FOR
DRUG INTERACTIONS
OACs are incompatible with treatments for many comorbidities including depression or arthritis. Amplatzer Amulet Occluder is an alternative to oral anticoagulants that can increase the risk of drug interactions.

Michael (AGE 75)
Michael has Non-Valvular Atrial Fibrillation (NVAF) and a CHA2DS2-VASc score of 5. He is reducing his stroke risk without increasing his risk for drug interactions.
NVAF patients with comorbidities should know that there is an alternative.
 </picture>
</picture>
ARE NOT ADHERING TO ROUTINE MONITORING
Adverse effects, the cost of Novel Oral Anticoagulants (NOACs), or the monitoring required when taking warfarin, often make compliance a challenge for patients. Amplatzer Amulet Occluder is an alternative to life-long oral anticoagulants that can lead to noncompliance.

Sarah (AGE 73)
Sarah has NVAF and a CHA2DS2-VASc score of 4. She is reducing her stroke risk without having to take OACs for the rest of her life.
NVAF patients who struggle with compliance should know that there is an alternative.
For educational purposes only; not real patient cases.
KEY FEATURES
Innovative Dual Seal Technology
The Amulet Occluder's innovative dual seal technology leverages a proven Amplatzer design that has been
trusted for over 20 years.2
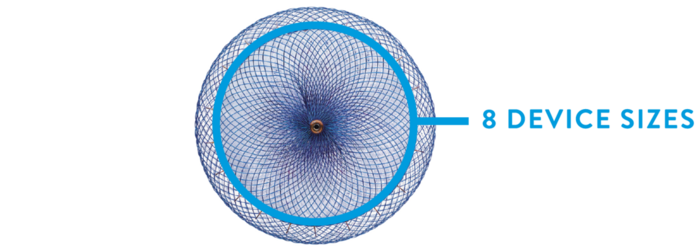
Widest range of sizes ensure best LAA fit
The Amulet Occluder offers 8 sizes and allows versatility to treat a broad range of LAA morphologies.
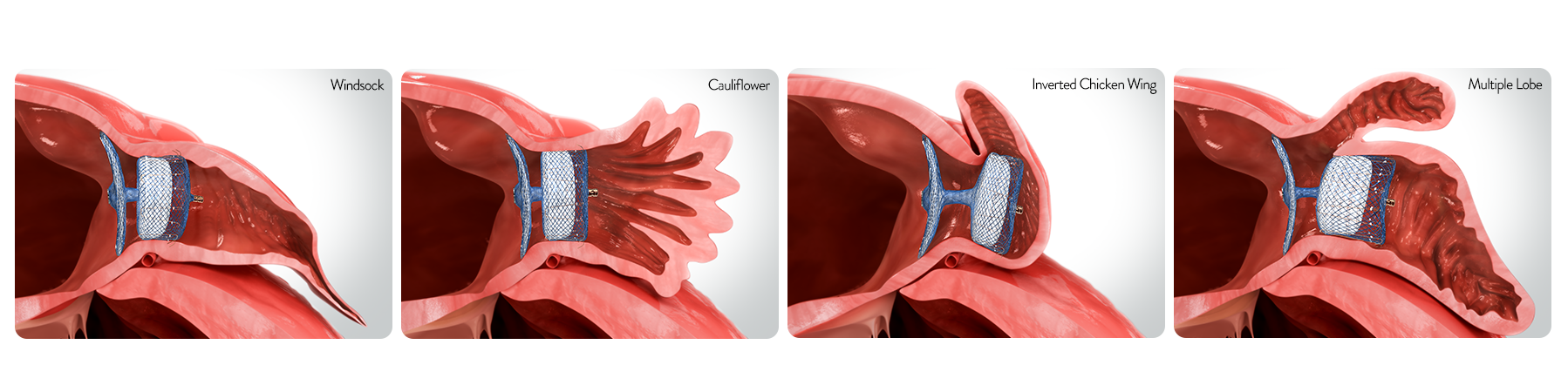
Treating the simplest to most complex morphologies
80%
of appendages have
more than 1 lobe10
NEARLY
50%
of appendages are chicken wing
anatomy which presents challenges
with a shallow landing zone11

Physician Education
Hear from physicians how the Amplatzer Amulet Occluder can benefit physicians and help patients live longer, fuller lives.
MAT-2008374 v9.0 | Item approved for OUS use only.