TRICLIP™ TRANSCATHETER TRICUSPID VALVE REPAIR SYSTEM
TriClip™ Transcatheter Edge-to-Edge Repair (TEER) offers a minimally invasive treatment option for patients with symptomatic, severe tricuspid regurgitation (TR) who are at high risk for surgery.1
TRICLIP™ TRANSCATHETER TRICUSPID VALVE REPAIR SYSTEM
TriClip™ Transcatheter Edge-to-Edge Repair (TEER) offers a minimally invasive treatment option for patients with symptomatic, severe tricuspid regurgitation (TR) who are at high risk for surgery.1
THE STANDARD OF
CARING FOR TR
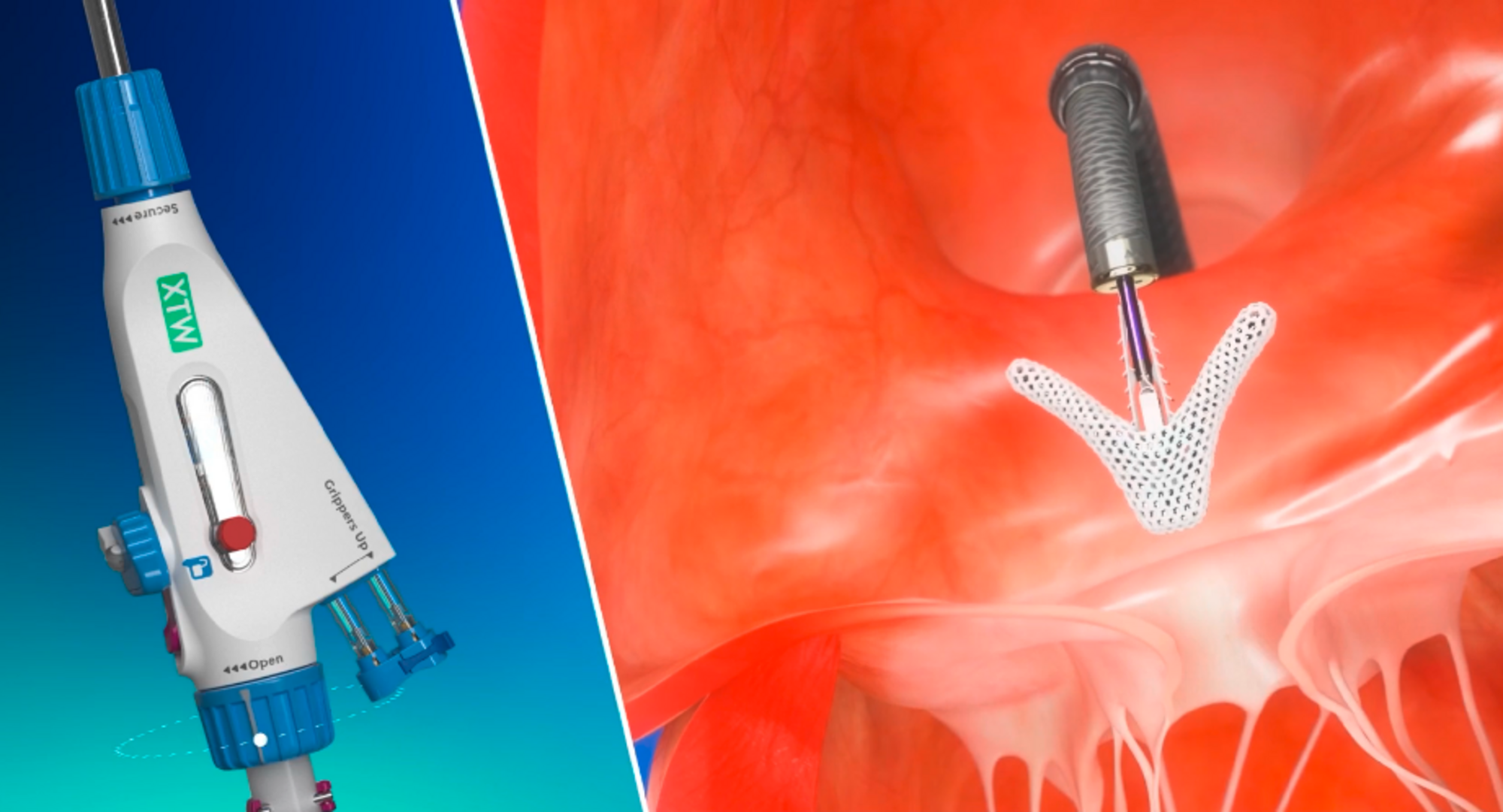
The TriClip Transcatheter Edge-to-Edge Repair (TEER) system is the only TEER device intentionally designed for the tricuspid valve. With stable navigation and precise delivery,2-5 TriClip TEER is the only tricuspid device proven to reduce risk of heart failure hospitalization and improve quality of life, relative to optimal medical therapy.6 In both randomized and real-world studies, TriClip TEER has proven:
- Exceptional safety profile9,10
- Maximized effectiveness in tricuspid regurgitation (TR) reduction6,7
- Life Changing Improvements to heart failure hospitalization risk and quality of life6,7,9,10
PATIENTS WHO BENEFIT
A state-of-the-art treatment option for patients at intermediate or greater risk for surgery
TriClip TEER is a low-risk,11 non-surgical treatment option for improving quality of life and functional status in patients with symptomatic severe tricuspid regurgitation, despite optimal medical therapy, who are at intermediate or greater risk for surgery and in whom transcatheter edge-to-edge valve repair is clinically appropriate and is expected to reduce tricuspid regurgitation severity to moderate or less, as determined by a multidisciplinary heart team.2
- Transcatheter beating heart procedure - no cardiopulmonary bypass
- Allows for real-time positioning and repositioning to optimize TR reduction
- Femoral venous access
- Can be used in a standard cath lab or hybrid room
- No pre-procedural CT required
- Fast recovery times; many patients go home the next day8
- Reduced HFH & QoL12
- Shortness of breath
- Peripheral edema
- Ascites
- Fatigue
- Declining exercise capacity
- Current treatment options have limitations
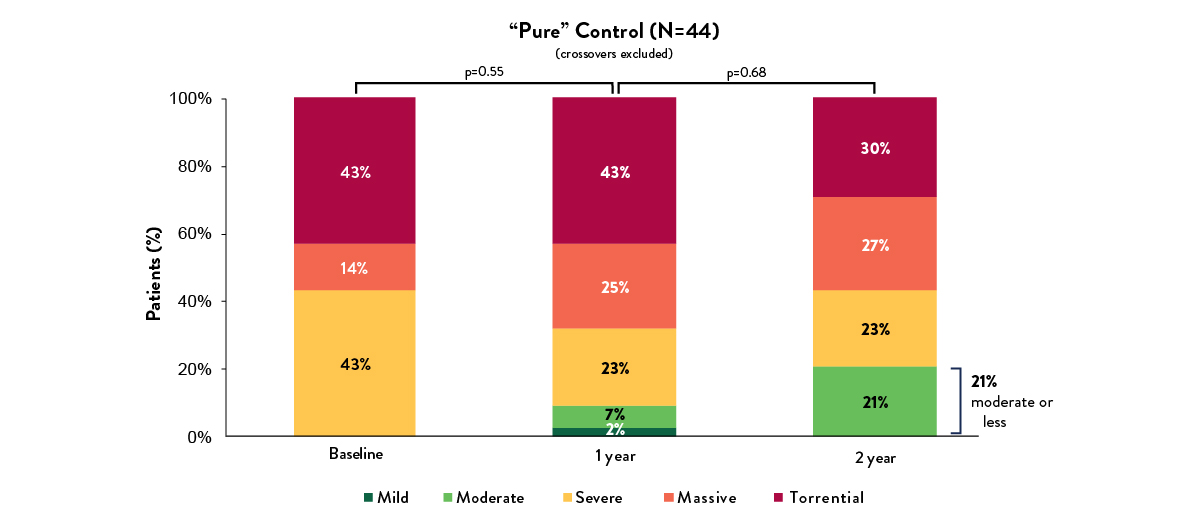
Medical therapy for TR has little impact on TR reduction – 2 year data6
66%of patients with severe functional tricuspid regurgitation die within 5 years of medical management13
Most patients receive medical management until right heart failure or end-organ dysfunction appears.14 - Surgery is high risk and often not optimal15,16
Surgery for TR is uncommon. Factors prohibiting surgery include:
- Limited clinical evidence15
- Multiple comorbidities15
- Advanced age15
- High rate of adverse events17,18
Long-term survival estimates for patients undergoing surgical tricuspid valve replacement or repair11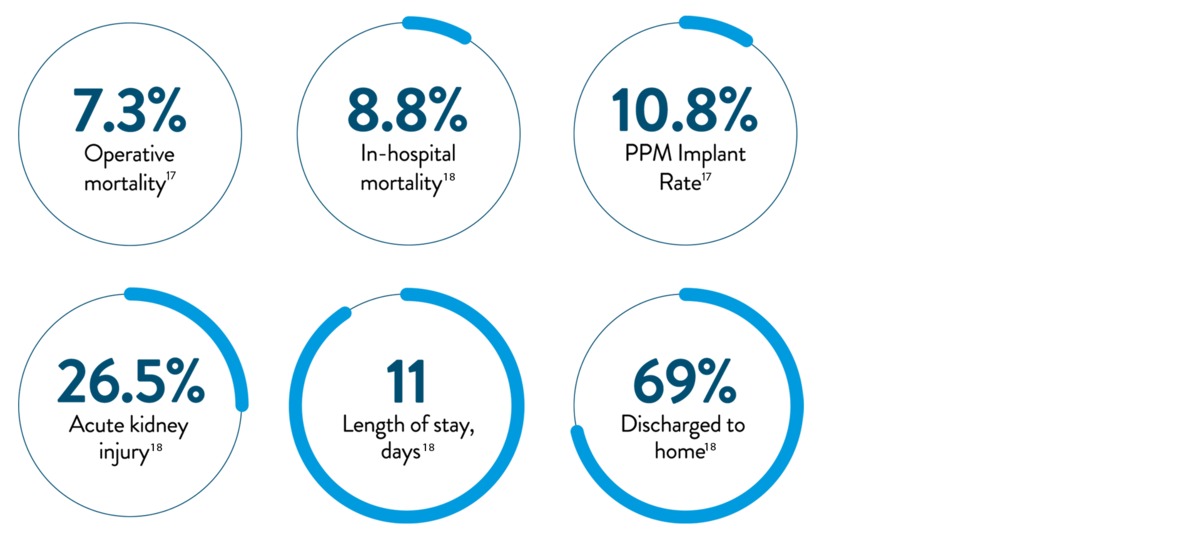
FEATURES

EXCEPTIONAL SAFETY9,10

MAXIMIZED EFFECTIVENESS6,7

LIFE-CHANGING IMPACT6,7,9,10
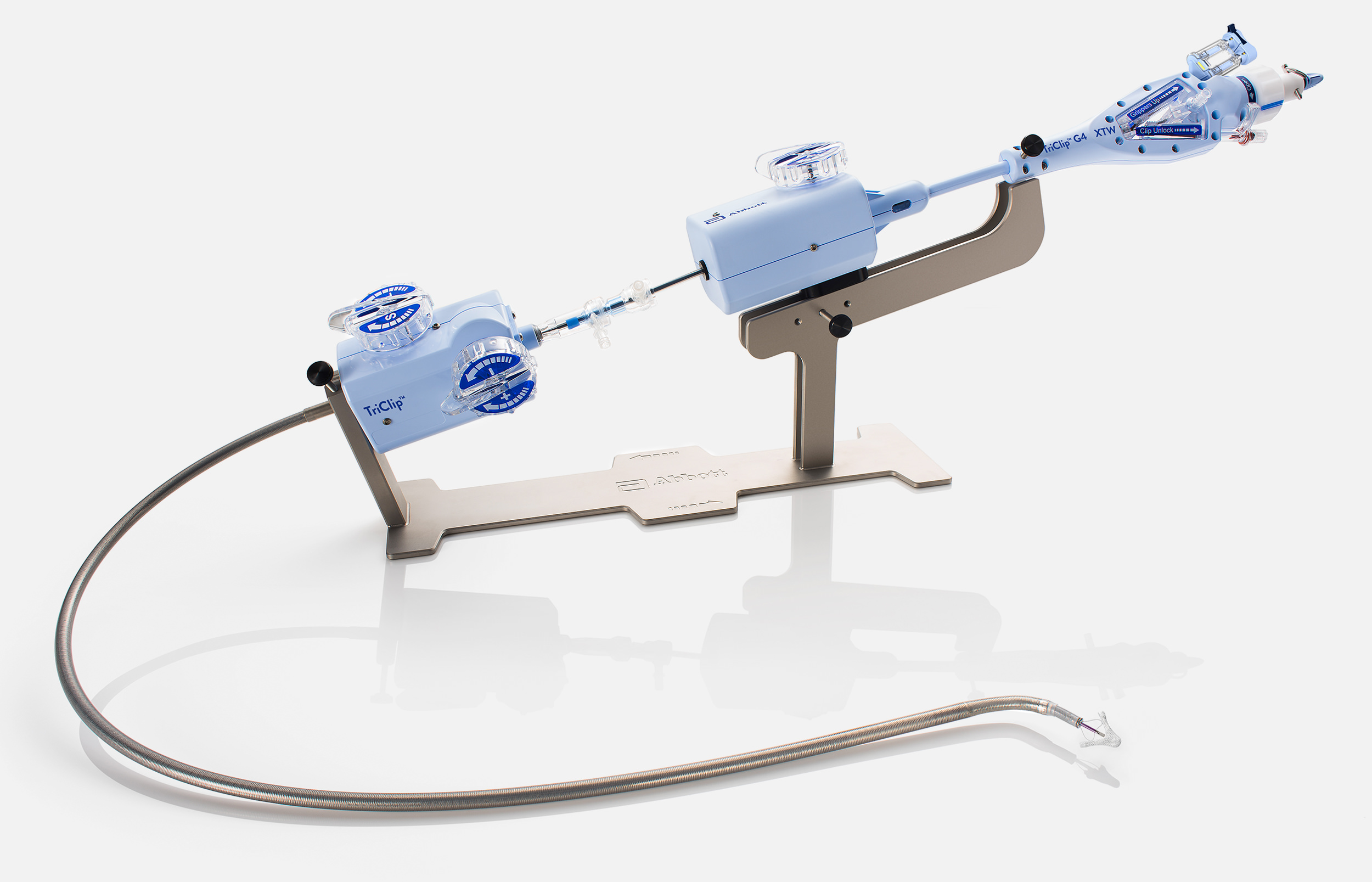
The TriClip™ G5 Transcatheter Edge-to-Edge Repair System empowers you with stable navigation and precise delivery2,5
for complex conditions.9,6
Delivery Catheter (DC) Handle
Handle has integrated gripper levers and reduced flush volume.
Arm Positioner
Hard stop within arm positioner in open direction.
Release Pin
Finger hold to pull and initiate clip deployment.
Lock Line Release Latch
Releases one end of lock line at time of deployment.
Lock Knob
Discrete lock and unlock positions and no blue line.
Delivery System (DS) Attach
Spring-loaded attachment slides into stabilizer rail. Replaces black screw.
Stabilizer
Stabilizer rail and handles to support the movement of the delivery system.
Guide Attach
Spring-loaded attachment with pins for insertion into stabilizer dock. Replaces black screws and the need to tighten screw to secure position.
Guide Attachment Braking System
Allows for adjustable guide positioning and holds guide catheter position. Replaces black screw.
Built-in DC Fastener
Rotation of fastener counterclockwise secures delivery catheter. Replaces black screw.
- Provides stability and precision during steering and positioning
- Multiaxis steering to enable navigation across all lines of coaptation
- Flexes and extends delivery catheter to steer down to the valve plane
- Enables movement in septal or lateral direction
- Straightens and curves guide for height adjustment above the valve
Designed for the tricuspid anatomy
- Provides adequate height over the valve
- Maintains coaxial position during steering and positioning
- Allows sweeping away from the septum to optimize delivery catheter while maintaining perpendicularity to the valve plane
- Designed for direct access to the tricuspid valve
- Confirm and optimize leaflet capture
- Implant and gripper line detachment
EASY HANDLING
- Quick, secure docking with spring-loaded attachments for easy connection
- Precise locking with binary lock knob to support consistent performance
- Built-in fastener for easy securement of the DC
PRECISE POSITIONING
- New guide attachment with braking system maintains desired positioning for enhanced control
- Controlled movement with redesigned stabilizer rail and handles
- Responsive new handle design capable of delivering 1:1 rotation
INTUITIVE DEPLOYMENT
- New lock knob offers easy and straightforward lock line release and removal
- Hard stop within arm positioner reduces dependence on visual indicators for more control
- Disconnect with ease using new release pin with finger hold to initiate deployment
Test(s) performed by and data on file at Abbott.
- Building on a legacy of unmatched TEER expertise19
TriClip™ implants use the same proven leaflet repair technology as our MitraClip™ Transcatheter Edge-to-Edge Repair (TEER) implants. TriClip TEER is the clip-based technology physicians know and trust, with a delivery system uniquely designed for the tricuspid valve.
TriClip implant features:1
- Elgiloy® implants with nitinol grippers
- Polyester cover designed to promote tissue growth
- Magnetic resonance conditional to 3 Tesla†
†Static magnetic field of 1.5 T or 3 T; maximum spatial gradient in static field of 4000 gauss/cm; maximum whole-body averaged specific absorption rate (SAR) of 2 W/kg for 60 minutes of continuous radiofrequency.
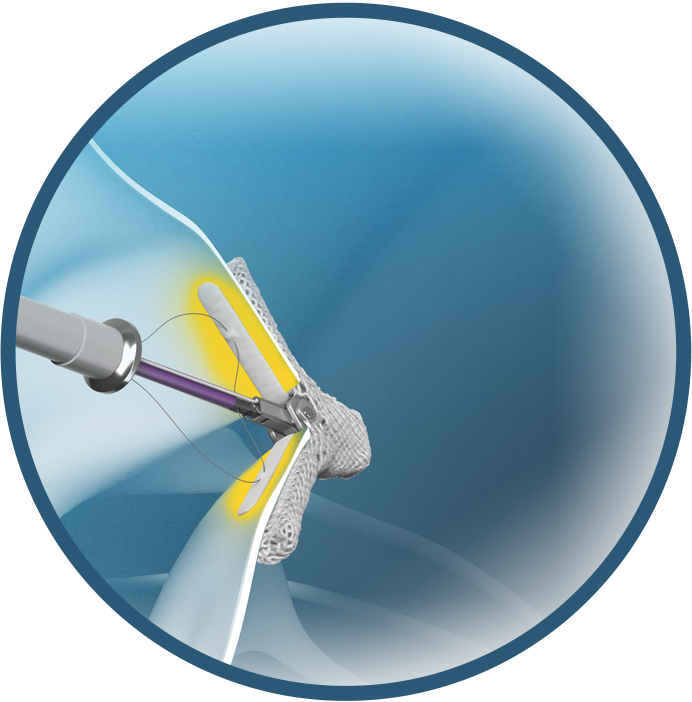
- Optimized leaflet capture
Optimized leaflet capture for consistent TR reduction with the TriClip G5 TEER System
- Wide grasp opening allows full leaflet insertion20
- Designed to distribute retention forces along the entire inserted leaflet length21
- Four or six rows of short frictional elements designed to provide atraumatic leaflet engagement21
- Broad range of sizes for tailored treatment
Four implant sizes are available for patient-specific therapy with the TriClip G5 TEER System.1

CLINICAL DATA
See the latest clinical data for the TriClip Transcatheter Tricuspid Valve Repair System
MAT-2006554 v11.0 | Item approved for OUS use only.


