MITRACLIP™ Transcatheter Edge-to-Edge Repair (TEER)
Uncompromising performance that sets the standard in mitral valve repair. With over 20 years of experience, extensive clinical evidence, and more than 200K patients treated worldwide.1
PATIENTS WHO BENEFIT
A better quality of life is possible for your patients with Mitral Regurgitation (MR)
The MitraClip G5 System is indicated for patients with symptomatic primary (degenerative) or secondary (functional) significant mitral regurgitation (MR ≥ 2+).
The catalyst for a significant evolution in MR guidelines
MitraClip is the first TEER (TMVr) therapy recommended by AHA/ACC/HFSA and ESC/EACTS guidelines and APSC recommendations for select primary and secondary MR patients.2-5,12
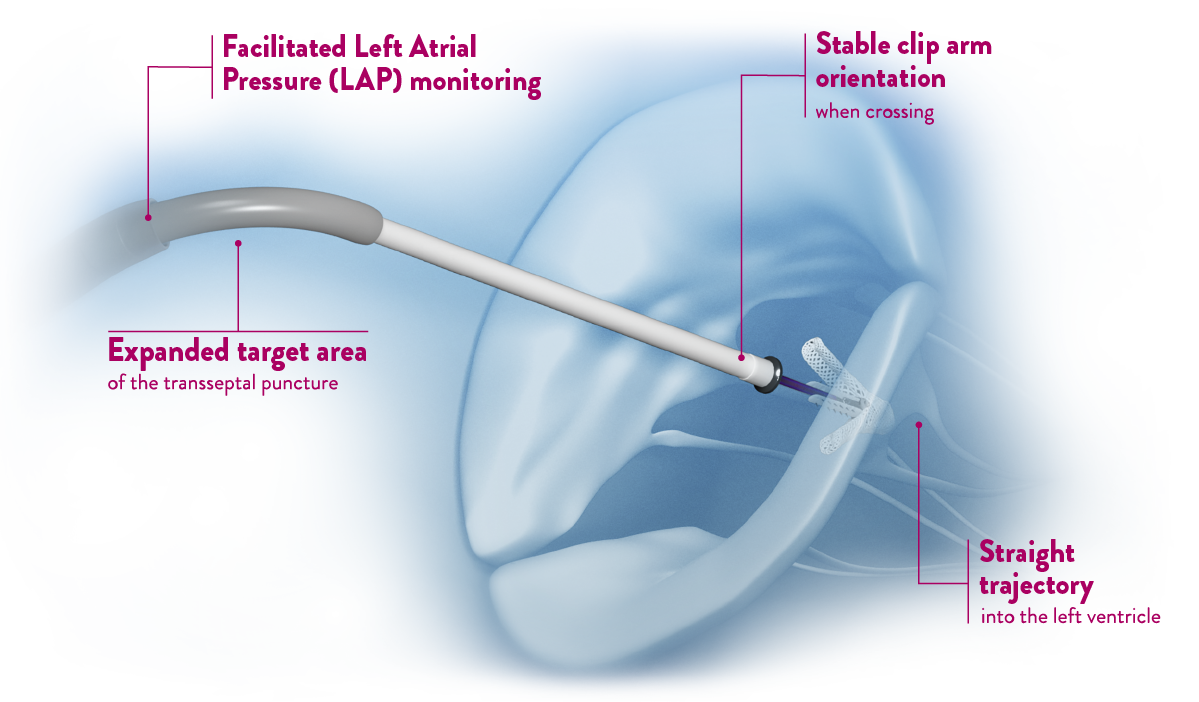
Severe MR is vastly undertreated
- Surgical intervention not offered or denied
49% of patients with symptomatic severe MR were not operated due to age, co-morbidities, or impaired LV.6 If left untreated, MR initiates a cascade of events leading to death, with 1-year mortality up to 57%7
IDENTIFYING PATIENTS FOR MITRACLIP THERAPY
MitraClip Transesophageal Echo (TEE)
Screening Guide
MitraClip Procedural Positioning and Imaging Guide
MitraClip Transthoracic Echo (TTE)
Screening Guide
FEATURES
MitraClip G5 TEER System
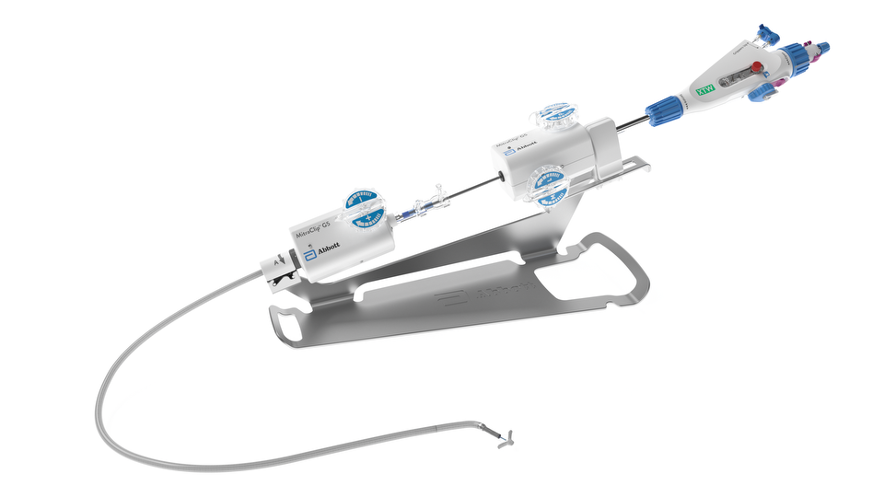
- Guide Attach
- DS Attach
- DC Fastener
- Lock Line Release Latch
- Lock Line Release Slider
Delivery Catheter (DC) Handle
Handle has integrated gripper levers and reduced flush volume.
Arm Positioner
Hard stop within arm positioner in open direction.
Release Pin
Finger hold to pull and initiate clip deployment.
Lock Line Release Latch
Releases one end of lock line at time of deployment.
Lock Knob
Discrete lock and unlock positions and no blue line.
Delivery System (DS) Attach
Spring-loaded attachment slides into stabilizer rail. Replaces black screw.
Stabilizer
Stabilizer rail and handles to support the movement of the delivery system.
Guide Attach
Spring-loaded attachment with pins for insertion into stabilizer dock. Replaces black screws and the need to tighten screw to secure position.
Guide Attachment Braking System
Allows for adjustable guide positioning and holds guide catheter position. Replaces black screw.
Built-in DC Fastener
Rotation of fastener counterclockwise secures delivery catheter. Replaces black screw.
Test(s) performed by and data on file at Abbott.
Easy Handling. Precise Positioning. Intuitive Deployment.
The MitraClip G5 system is designed to support procedural control and efficiency in mitral valve repair. With thoughtfully integrated enhancements, the system facilitates connection, movement, and disconnection with minimal complexity. Key updates include refined attachment mechanisms and securement features, contributing to a more streamlined procedural experience.
Learn more about the additional benefits of MitraClip TEER.
- Broad range of sizes for tailored treatment8-11
MitraClip TEER offers 4 Clip sizes to help tailor repair based on patient mitral valve anatomy.
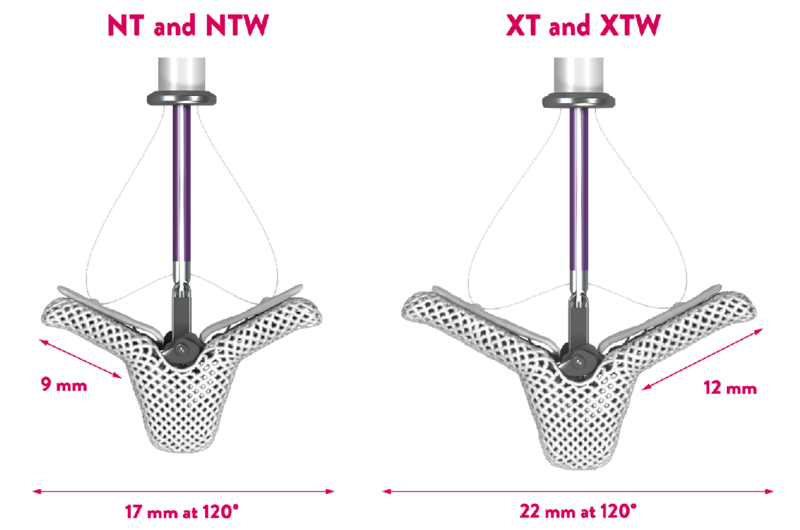
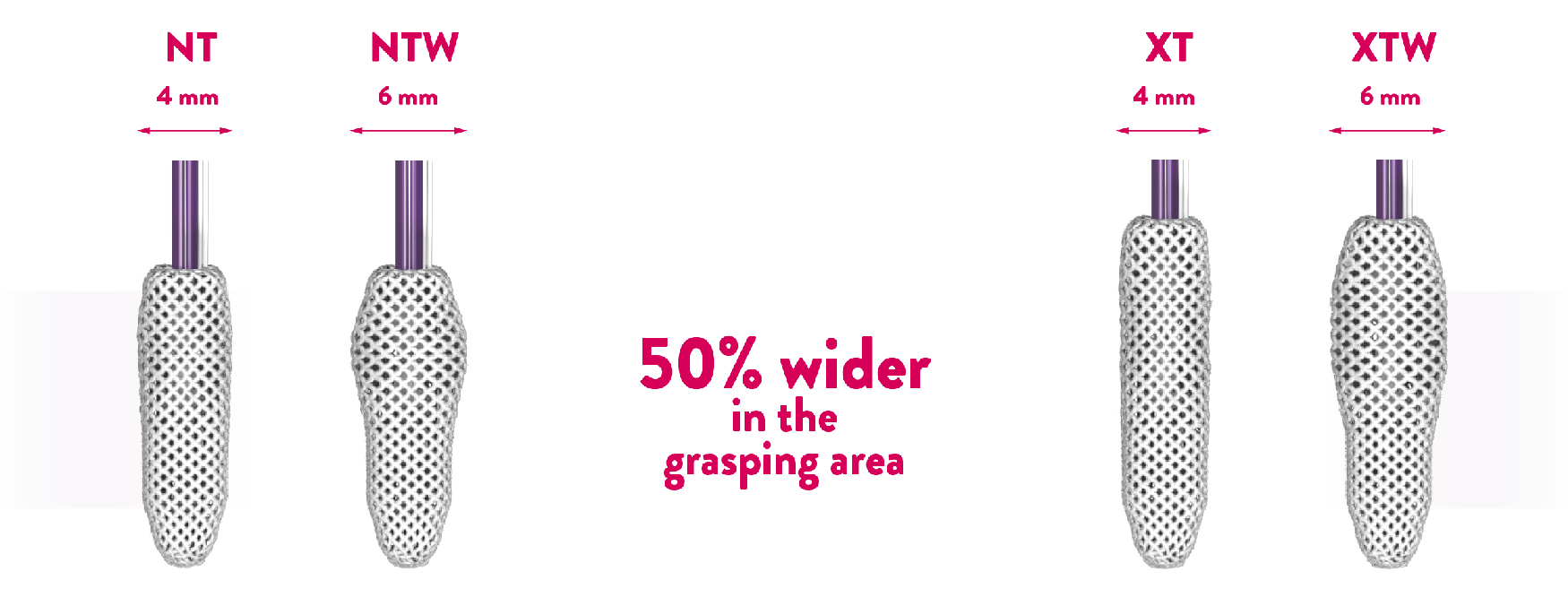
The NTW and XTW clips have a 50% wider grasping area than the NT and XT clips respectively. They are designed to further reduce regurgitant volume reduce MR with the implantation of a single clip.**
- Treat more patients with more options8-11
MitraClip successfully treats a broad range of valve anatomies in real world use8-11
Significant and durable MR reduction achieved in patients with complex anatomies11
90.3% of complex subjects achieved MR ≤1+ at 1 year.11
Ability to choose clip size based on each MV anatomy8,10
CLIP SELECTION CONSIDERATIONS FAVORS
NTWFAVORS
NTFAVORS
XTWFAVORS
XTLeaflet length < 9mm + + Leaflet length ≥ 9mm + + Broad jet + + Smaller valve + Larger valve + + + MitraClip TEER clip selection recommendations were based on the initial clinical experience of an expert panel of physicians using the 4 Clip sizes of MitraClip G4.8,10 The implant device for the MitraClip G4 and the MitraClip G5 TEER Systems is identical.
The EXPAND G4 real world study results further demonstrated clip selection preference and associated outcomes:10

FOR PATIENTS WITH PMR
XTW was used most often and achieved favorable MR reduction, particularly in patients with longer leaflets, large prolapse or wider jets, calcified leaflets or annulus and Barlow’s or bileaflet prolapse

FOR PATIENTS WITH SMR
NTW and XTW were used evenly across anatomies and achieved favorable MR reduction

FOR ALL MR ETIOLOGIES
NT and XT were used more frequently in multiple-clip cases and improved MR reduction
- More options to confirm and optimize leaflet grasping with Controlled Gripper Actuation (CGA)1,**
Controlled Gripper Actuation (CGA) provides the option to grasp leaflets simultaneously or independently, enabling the ability to confirm and optimize leaflet grasp.
New gripper
levers
Simultaneous
graspingIndependant
grasping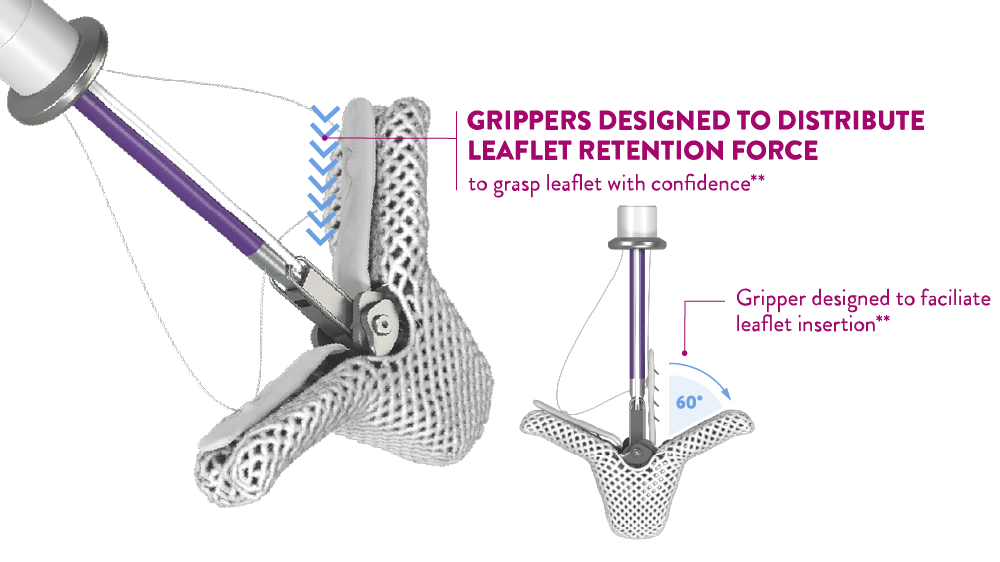
- Predictable procedure experience**
The innovative Clip Delivery System is a highly maneuverable delivery catheter that is used to implant the MitraClip via a Steerable Guide Catheter.
Precision and control from delivery system specifically designed for the mitral valve**
MAT-2010221 v8.0 | Item approved for OUS use only.
