From transcatheter and surgical valve therapies to structural interventions,
we’ve built a broad structural heart portfolio across the spectrum to restore health and improve quality of life for patients with structural heart disease.
YOUR PARTNER OF CHOICE
FOR STRUCTURAL HEART SOLUTIONS
Abbott offers a broad portfolio of structural heart solutions for an array of conditions. We invest in creating innovative technologies and advancing science, with the ultimate goal of helping patients achieve better health and improved quality of life. With our legacy of innovation, product diversity, safety, and performance, Abbott is your partner for structural heart therapies.
Some of the ways Abbott demonstrates its leadership in the industry include:
- Established leader in transcatheter edge-to-edge repair (TEER)* with MitraClipTM1
- Offering TriClipTM, the first and only TEER device, to date, designed specifically for the tricuspid valve
- Offering among the broadest occluder portfolios, with 25+ years of leadership1
- Demonstrating exceptional hemodynamics with tissue valves, and an impressive 40-year legacy with mechanical valves
- Providing outstanding & competitive clinical outcomes physicians expect from the latest generation TAVI system
200K+
patients treated
Established leader in
transcatheter mitral valve repair
with MitraClipTM 1
25+
years of leadership
in occluders
We offer a broad portfolio
with PFO, LAA, PDA, septal,
and muscular VSD occluders
40
year legacy
We have an impressive 40-year legacy with mechanical valves and have demonstrated exceptional hemodynamics with tissue valves
*TEER is also referred to as TMVr (transcatheter mitral valve repair)
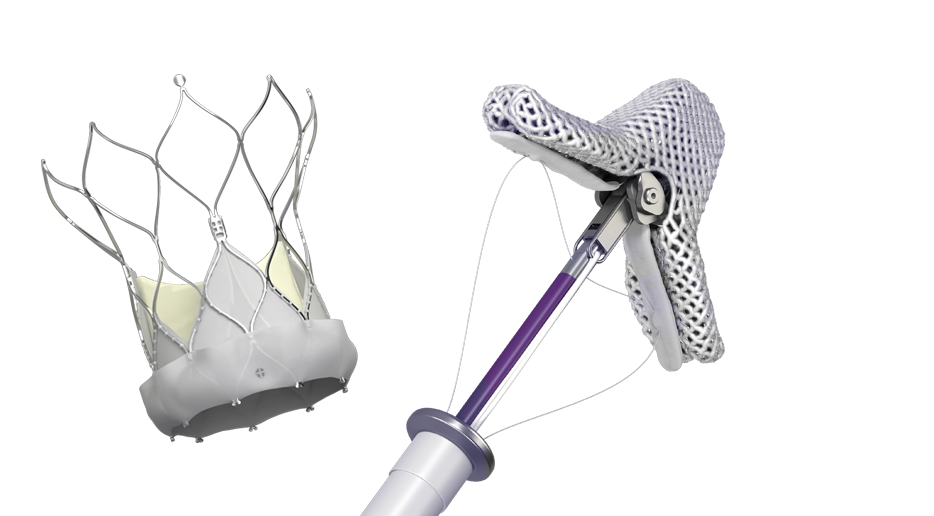
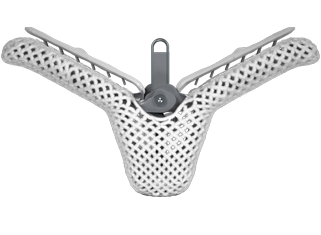
TRANSCATHETER VALVE SOLUTIONS
BUILT TO HELP
PATIENTS RECLAIM
THEIR LIVES
Our minimally invasive transcatheter solutions are proven to safely, effectively repair or replace heart valves for patients and deliver excellent outcomes.
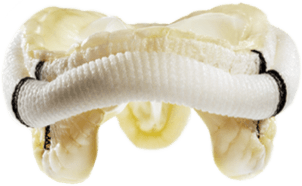
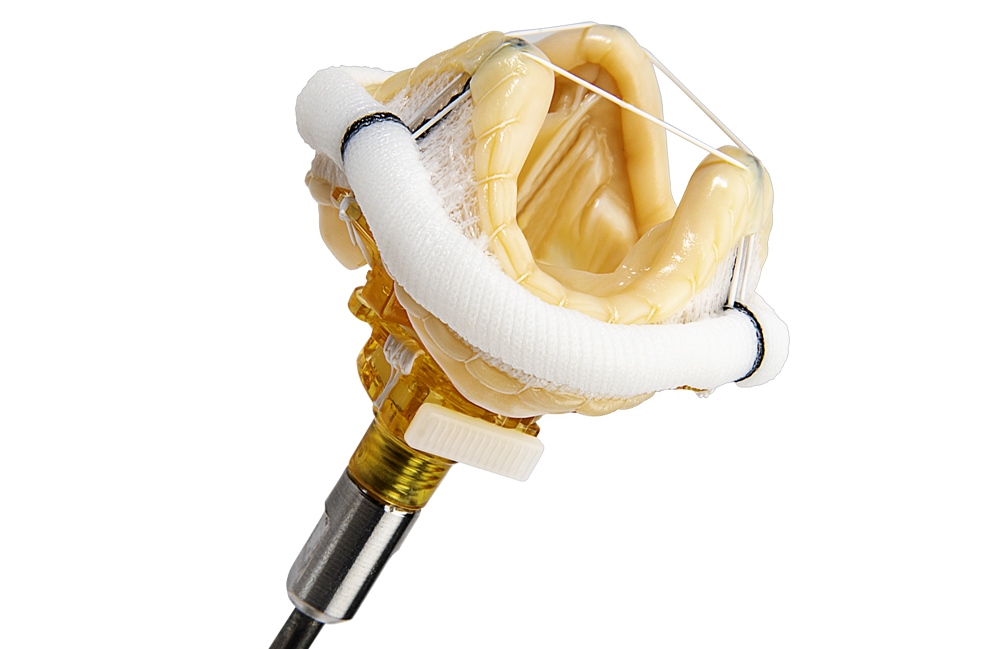
SURGICAL VALVE SOLUTIONS
DESIGNED TO REBUILD HEALTHIER HEARTS
Our comprehensive surgical valve portfolio delivers long-term durability and performance, exceptional hemodynamics, and streamlined implantability.

SURGICAL VALVE SOLUTIONS
DESIGNED TO REBUILD HEALTHIER HEARTS
Our comprehensive surgical valve portfolio delivers long-term durability and performance, exceptional hemodynamics, and streamlined implantability.
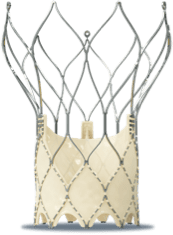
STRUCTURAL INTERVENTIONS
BUILT FOR THE AGES.
DESIGNED FOR
COMPLETE CLOSURE.1
Our occluders are built to close an opening in patients’ hearts—saving and restoring lives by providing closure with confidence.


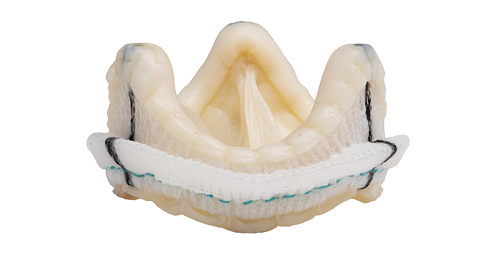
STRUCTURAL INTERVENTIONS
BUILT FOR THE AGES.
DESIGNED FOR
COMPLETE CLOSURE.1
Our occluders are built to close an opening in patients’ hearts—saving and restoring lives by providing closure with confidence.
MAT-2000632 v7.0 | Item approved for OUS use only.