AMPLATZER™ TALISMAN™PFO OCCLUDER
Effective PFO closure is made easier with the Amplatzer™ Talisman™ PFO Occluder.1 For patients who have experienced a PFO-associated stroke, clinical data show they can benefit from PFO closure, with this minimally invasive procedure significantly reducing the risk of recurrent ischemic stroke and offering an excellent safety profile.2-4
THE PIONEER
IN PFO CLOSURE1
The Amplatzer™ Talisman™ PFO Occluder set the standard as the first device supported by positive PFO trial results, developed specifically for patent foramen ovale (PFO) closure to reduce the risk of recurrent ischemic stroke.2 Our clinical evidence is unmatched, thanks to the largest-ever trial for PFO closure, boasting 5,810 patient-years of data.2
Today, with over 250,000 patients treated worldwide and a legacy spanning over 25 years, it is the #1 device worldwide.1
The PFO Occluder trusted by thousands of physicians around the world1
250K+
Global implants1
We set the standard
- Pioneered treatment with a PFO-specific device
- Available in over 80 countries around the world
5,810
PATIENT-YEARS OF DATA2
We raise the bar
- With the landmark RESPECT trial, we had the most extensive patient follow-up, almost 2x more than other PFO trials
- RESPECT was also the only trial to include patients on anticoagulation therapy, a real-world cross-section of patients
990
PATIENTS IMPLANTED WITH DEVICE IN RCTS2,4-8
We demonstrate excellence
- ZERO device erosions, thrombus, embolization events or wire frame fractures in 6 published trials with 990 patients
- 94.2% effective closure rate at 6 months
PATIENTS WHO BENEFIT
PFO closure reduces the risk of another stroke in patients 18-60 by 59% vs. medical management alone.9
For patients who have experienced a PFO-associated stroke, the thought of a recurrence can generate anxiety. Determining and delivering the most effective treatment soon after a stroke is critically important to provide these patients peace of mind.
For more patient and caregiver resources and to learn more, head to www.PFOStroke.com
In a real-world analysis of patients >60 years with presumed PFO-associated stroke, patients
who received the Amplatzer Talisman PFO Occluder saw a 38% relative risk reduction of recurrent ischemic stroke over non-closure patients.10
This testimonial relates an account of an individual’s response to the treatment. This patient’s account is genuine, typical and documented. However, it does not provide any indication, guide, warranty or guarantee as to the response other persons may have to the treatment. Responses to the treatment discussed can and do vary and are specific to the individual patient.
KEY FEATURES
Often Imitated,
Never Matched1,11
Unique design features set the Amplatzer Talisman PFO Occluder apart from the rest.
*Only the 18 mm size is not asymmetric.
†Effective closure.
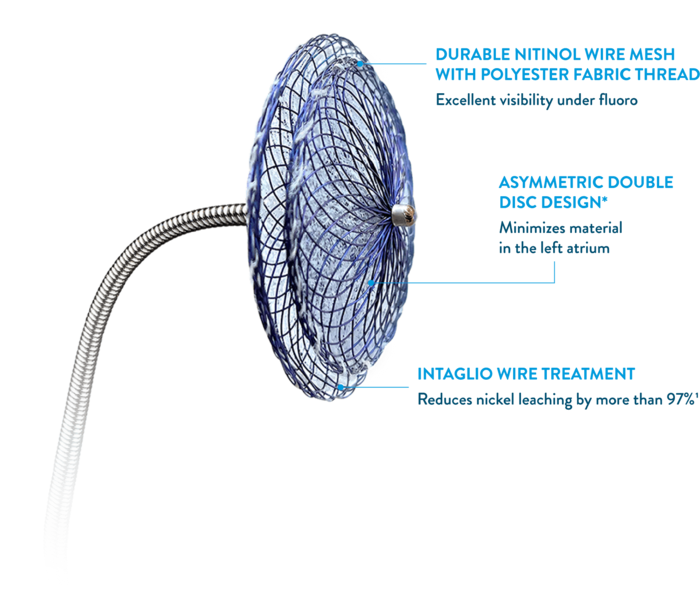
*Only the 18 mm size is not asymmetric.
Minimizing Complexity in PFO Closure
SIMPLIFIED
PREP
Occluder comes assembled to the flexible Trevisio™ delivery cable, ready to use

RECAPTURABLE AND REPOSITIONABLE
Self-expanding discs align to the PFO without an additional “locking” step and can be adjusted for ideal placement
LOW-PROFILE
DELIVERY
8 F and 9 F introducer sheaths enable treatment of patients with smaller vasculature

Physician education
Hear the latest on real world outcomes with the Amplatzer Talisman PFO Occluder in patients >60 years
MAT-2010605 v7.0 | Item approved for OUS use only.