AMPLATZER™ DUCT OCCLUDERS
Designed for flexibility, our innovative duct occluder options conform to a variety of duct sizes while achieving complete patent ductus arteriosus (PDA) closure from a pulmonary or aortic approach.
INDUSTRY-LEADING AMPLATZER™
PDA OCCLUDERS1
The Amplatzer™ family of patent ductus arteriosus (PDA) closure occluders provides proven closure for a wide variety of patient morphologies. These devices share several Amplatzer characteristics:
- Provide secure positioning in the ductus
- Achieve complete closure
- Promote tissue in-growth
- Provide flexibility and conformability
PATIENTS WHO BENEFIT
PDA Closure in Infants
Constriction of the ductus arteriosus is a critical step in postnatal circulatory transition. If the ductus remains open, PDA occurs, resulting in left-to-right shunting that can create significant challenges, especially in premature infants.
Challenges include2:
- Pulmonary over-circulation in lungs that are already under duress
- Systemic hypoperfusion
As the only PDA closure solution indicated for premature infants ≥ 700 grams + ≥ 3 days old and proven to deliver safe and effective closure, Amplatzer Piccolo™ Occluder offers new opportunities to care for a wider range of patients than ever before.3
PDA Closure Guidelines in Adults
PDA closure is recommended if left atrial or left ventricular enlargement is present and attributable to the PDA with left-to-right shunt, pulmonary artery systolic pressure < 50% systemic, and pulmonary vascular resistance less than one-third systemic.4
PDA closure may be considered in the presence of a net left-to-right shunt if pulmonary artery systolic pressure is ≥ 50% systemic, and/or pulmonary vascular resistance is greater than one-third systemic.4
Transcatheter PDA closure—eg, with an Amplatzer ductus occluder—is the standard of care in most cases.5
KEY FEATURES
Amplatzer Piccolo™ Occluder:
For PDA Closure in the smallest patients
The Amplatzer Piccolo™ Occluder, which delivers proven PDA closure for patients 700 grams and up, provides the strength to occlude small ducts, while minimizing protrusion into surrounding pulmonary artery and descending aorta.3
Features include:
- Low-profile retention discs and end screw are designed to minimize protrusion into the aorta and pulmonary artery
- Symmetrical design for either pulmonary or aortic approach
- Tightly woven mesh to minimize residual shunt immediately after positioning
- Flexible delivery wire for ease of deployment
- Ability to be easily recaptured and redeployed for optimal placement8
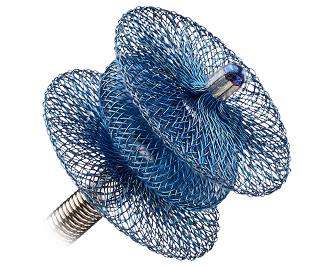
Amplatzer™ Duct Occluder: For large PDA closure
Backed by over 15 years of performance in the U.S.,1 the Amplatzer™ Duct Occluder can accommodate large PDAs with a single device—which can aid in minimizing the complexity of the procedure.
Features include:
- A conical shape that can help conform to tapering ducts
- A retention skirt to prevent embolization
Treatment range:
- Ductus diameter: 3.0-12.0 mm
- Ductus length: > 3.0 mm*
*The Amplatzer Duct Occluder can be used in any length defect as long as the device does not extend more than 3 mm into the pulmonary artery or does not occupy over half of the pulmonary artery lumen.

Amplatzer™ Duct Occluder II:
Versatility to conform to a variety of PDAs
The Amplatzer™ Duct Occluder II is engineered using 2 articulating discs and multi-layered mesh that conforms to most classifications of PDAs.
Features include:
- Symmetrical design so it can be introduced via pulmonary or aortic approach6
- 6 planes of occlusion for full, cross-sectional coverage7
- Fabric-free design allows for delivery through a low-profile catheter—while still achieving a very high occlusion rate6
Treatment range:
- Ductus diameter: 2.5-5.5 mm
- Ductus length: > 5.0-12.0 mm

MAT-2201732 v2.0 | Item approved for OUS use only.