AMPLATZER™ VENTRICULAR SEPTAL DEFECT OCCLUDERS
Ventricular septal defects (VSDs) are the most commonly found congenital heart defect.2 Our Amplatzer™ VSD portfolio includes occluders specifically designed to close complex VSDs in patients considered to be too high risk for standard surgical repair.
THE PIONEER IN VENTRICULAR SEPTAL OCCLUDERS1
The Amplatzer line from Abbott not only pioneered structural heart defect occluders, it also retains a leadership position in structural heart innovation and treatment worldwide.1 Amplatzer™ VSD Occluders have a record of safety and effectiveness for more than 20 years.
A ventricular septal defect (VSD) is a hole in the septum between the ventricles and is the most common type of heart malformation.2 A VSD allows blood from the left ventricle to pass through into the right ventricle, creating extra stress to the right ventricle in order to keep up with the additional blood flow. Patients with a VSD carry an increased risk of infection to the heart walls and valves.
Types of VSD
There are 4 types of VSDs:
- Perimembranous
- Muscular
- Conal, or subpulmonary
- Inlet
Amplatzer options for Ventricular Septal Defect closure
Abbott offers two devices for transcatheter VSD closure, the Amplatzer Muscular VSD Occluder and the Amplatzer Post-MI Muscular VSD Occluder. Like many Amplatzer occluders, these devices:
- Are self-expandable, double-disc devices
- Are made of nitinol wire mesh with interwoven polyester substrate to promote occlusion and tissue in-growth
- Can be recaptured and redeployed if necessary for precise placement
- Are designed for complete closure
- Have a symmetrical design that allows for a venous or arterial delivery approach
- Have a connecting waist that corresponds to the size of the VSD*
*With the condition that the implanter chooses the appropriate device size.
PATIENTS WHO BENEFIT
The importance of treating patients with VSD
*This testimonial relates an account of an individual’s response to the treatment. This patient’s account is genuine, typical and documented. However, it does not provide any indication, guide, warranty or guarantee as to the response other persons may have to the treatment. Responses to the treatment discussed can and do vary and are specific to the individual patient.
After receiving the Amplatzer™ VSD Occluder
Jozef, amazed at his energy and ability to walk
long distances again, said:
“I feel joy deep inside me—inside my heart.”
Amplatzer Muscular VSD Occluders
The Amplatzer Muscular VSD Occluder is indicated for use in patients with a complex VSD of significant size to warrant closure—large volume left-to-right shunt, pulmonary hypertension, and/or clinical symptoms of congestive heart failure—in patients who are considered to be at high risk for standard transatrial or transarterial surgical closure based on anatomical conditions and/or based on overall medical condition.
Benefits: Ease of transcatheter closure of VSD in patients
“The benefits of avoiding [cardiopulmonary] bypass are intuitive, and the relative ease of placement makes this procedure ultimately attractive.”3
- When closure should be performed
Both transcatheter and surgical closure are options for patients with moderate to large VSDs. In particular, physicians should perform VSD closure when the patient has2:
- Left ventricular (LV) volume overload and hemodynamically significant shunts, with pulmonary-to-systemic blood flow ratio (Qp/Qs) of 1.5 or higher*
- Worsening aortic regurgitation (AR) caused by perimembranous VSD
In patients whose VSD is not repaired, there is an increased risk of infective endocarditis (IE), usually involving the tricuspid and pulmonary valves.*Assuming that pulmonary artery (PA) systolic pressure is less than 50% systemic and pulmonary vascular resistance is less than one-third systemic.
- Treatment for Post-Myocardial Infarction Muscular VSD is critical
A post-myocardial infarction muscular VSD is a life-threatening complication that often leads to cardiogenic shock.4 Notably, medical management alone is associated with a grim prognosis of a 94% mortality rate at 30 days.5
The Amplatzer Post-infarct Muscular VSD Occluder is a percutaneous transcatheter occlusion device intended for closure of post-myocardial infarction muscular VSDs in patients who are not satisfactory surgical candidates.6

Due to his VSD, Jozef developed endocarditis, which in turn led to heart failure and kidney failure. After receiving an Amplatzer VSD Occluder, Jozef, astonished at his symptom improvement, noted “I feel joy deep inside me—inside my heart.”
KEY FEATURES
Choose between two Amplatzer devices for
Muscular VSD Closure
Abbott’s 2 muscular VSD occluders allow for either a femoral or an arterial delivery.
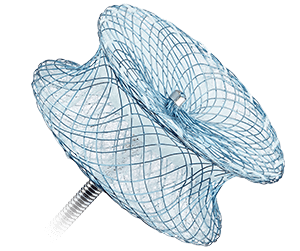
Amplatzer™ Muscular VSD Occluder
- Is designed for muscular VSD closure in patients considered to be high risk for standard surgical repair
- Has a 7 mm waist length to accommodate the thickness of the muscular septal wall
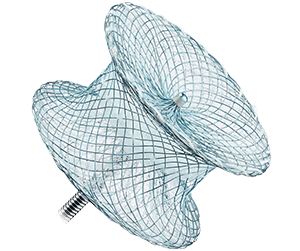
Amplatzer™ Post-infarct Muscular VSD Occluder
- Is designed for the damaged muscular tissue in the septal wall of patients who have had a myocardial infarction
- Has a 10 mm waist length to accommodate the damaged tissue that can occur with septal wall rupture
MAT-2008370 v6.0 | Item approved for OUS use only.