EPIC™ MAX
AORTIC STENTED TISSUE VALVE CLINICAL DATA
The Epic™ valve platform has a decades-long history of safety and strong clinical outcomes. Epic Max has an optimized design to further improve valve blood flow.
EPIC™ MAX
AORTIC STENTED TISSUE VALVE CLINICAL DATA
The Epic™ valve platform has a decades-long history of safety and strong clinical outcomes. Epic Max has an optimized design to further improve valve blood flow.
BUILT ON THE EPIC™PLATFORM THAT HAS STOOD THE TEST OF TIME
AT 10-YEARS
Over 11,000 Patients
94.6%
Epic Supra Valve
Freedom From All-Cause
Reintervention2
Over 1,900 Patients
96.3%
Epic Supra Valve
Freedom from SVD
(Structural Valve Degeneration)
All patient age3
COMMITED TO VALVE-IN-VALVE* FOLLOW-UP DATA
3-YEAR OUTCOMES OF VALVE-IN-VALVE INTERVENTION IN A U.S. POPULATION
>95.0%
Epic™ Supra Valve
Patients Experienced Freedom from Valve
Intervention4
OPTIMIZE OUTCOMES WITH EXCELLENT HEMODYNAMICS
ESTIMATED MEAN PRESSURE GRADIENT AT 1 YEAR FOR EPIC MAX (mmHg)1
MEAN PRESSURE GRADIENT PREDICTED BASED ON MATCHED STENT ID FOR BIOCOR/EPIC™ VALVES.
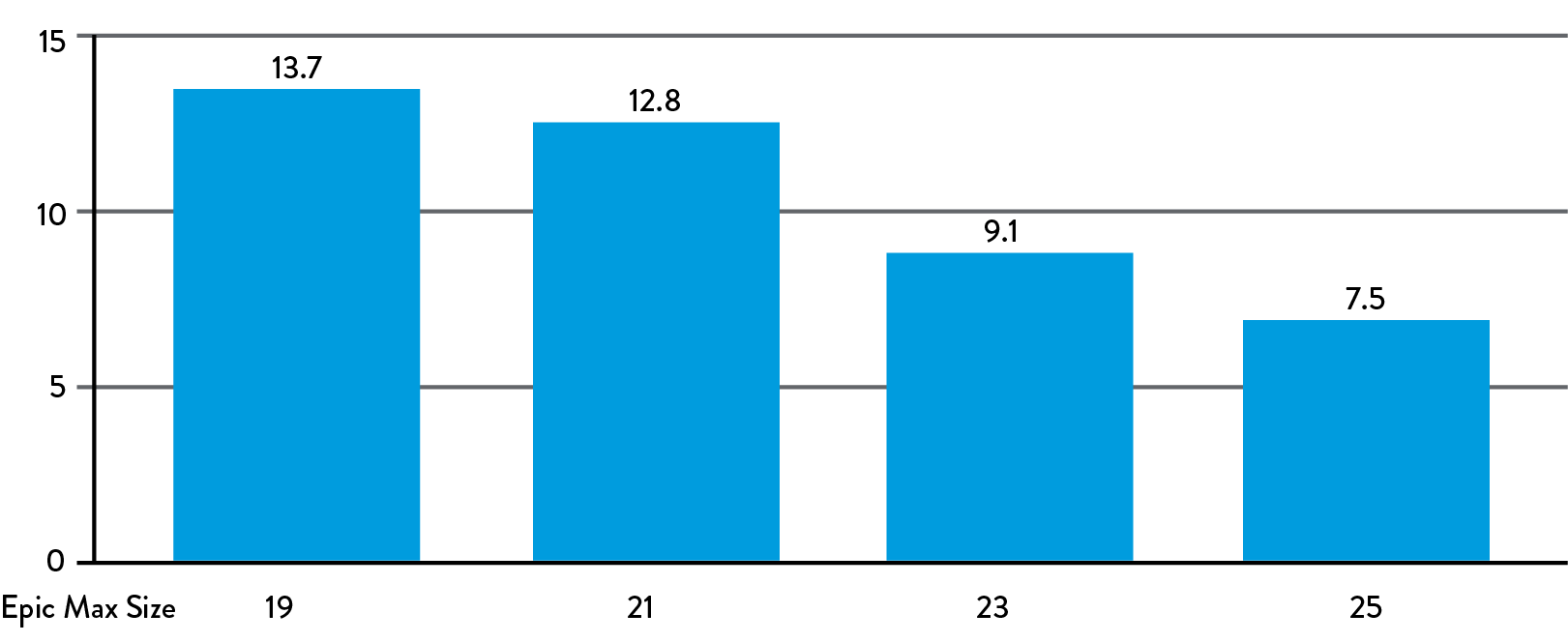
Maximizing the internal orifice provides large effective orifice areas (EOAs) and low gradients to reduce the burden on the heart and contribute to better patient outcomes.
*The safety and effectiveness of valve-in-valve procedures in an Epic™ Max valve has not been established
DOWNLOAD THE EPIC
MAX BROCHURE
MAT-2400567 v1.0 | Item approved for OUS use only.