BETTER EVIDENCE TO BUILD CONFIDENCE
Abbott provides a robust body of clinical evidence encompassing clinical data and outcomes from tens of thousands of patients across our structural heart portfolio of products.*
IN-DEPTH DATA ABOUT STRUCTURAL HEART PRODUCTS
With our Clinical Insights and Bulletins you can take a deeper dive into clinical outcomes related to structural heart products. Designed for ease of viewing, these pieces give you a broader perspective about study outcomes along with product safety and performance.
Transcatheter Edge-to-Edge Repair

TriClip™ for TEER
Read moreReducing heart failure hospitalization and empowering patients to live their best lives
TAVI

Navitor™ TAVI System: Clinical Insights
Read moreDevice design and favorable 5-year durability reinforce Navitor™ TAVI long-term outlook
Navitor™ Vision Valve: PVL Sealing by Unique Design
Read more0% Moderate to Severe PVL With Navitor™ Active-Sealing Cuff

Navitor™ Valve: Small Aortic Annuli
Read moreSingle-Digit Mean Gradients With The Self-Expanding Navitor™ Valve

Navitor™ TAVI System: Minimizing PPI Risks
Read morePPI Rates of 10% or Lower Using Best Implant Practices

Navitor™ TAVI System: Real-World Experience
Read more10.6% New PPI With Navitor™ Vision Valve After 20 Procedures
Structural Interventions
EMERGE LAA Post-Approval Study
Read moreOne-year real-world outcomes with the Amplatzer Amulet LAA Occluder in the U.S.
EMERGE LAA Post-Approval Study
Read moreConcomitant Catheter Ablation and LAA Occlusion with the Amplatzer™ Amulet™ LAA Occluder in the U.S.*

EMERGE LAA Post-Approval Study
Read moreHRS 2024: Feasibility of Amulet Occluder Implantation after failed LAA Occlusion attempt with Single Lobe Device.

EMERGE LAA Post-Approval Study
Read moreAF Symposium: Late Breaking Clinical Trial Emerge LAA Post-Approval Study

Amplatzer™ Amulet™ LAA Occluder
Read moreLAAO STROKE PREVENTION: Device-Related Factors Considerations
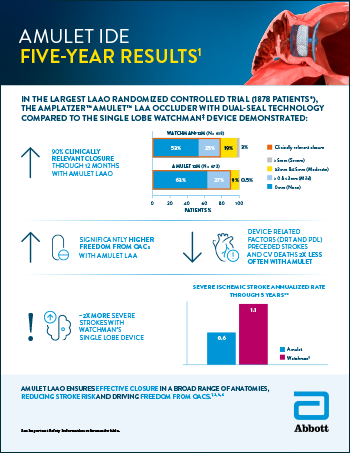
Amulet™ IDE 5-Year Results
Read moreThe Amplatzer™ Amulet™ LAA Occluder with Dual-Seal Technology compared to the Single Lobe Watchman‡ Device Demonstrated.
Surgical Valve Solutions
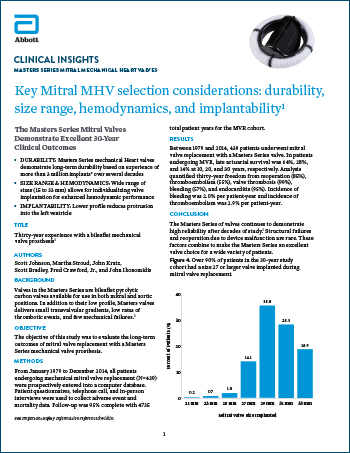
Masters Series Mitral Mechanical Heart Valves
Read moreKey Mitral MHV Selection Considerations: Durability,
Size Range, Hemodynamics and Implantability1
Epic™ Mitral Stented Tissue Valve with Linx™ AC Technology
Read moreLong-Term Outcomes and Freedom from Calcification

Epic™ Mitral and Epic™ Supra Stented Tissue Valves
Read moreThree-Year Outcomes of Valve-in-Valve Intervention within the Epic™ Supra and Epic™ Mitral Valves in a Medicare Population

Epic™ Supra Aortic Stented Tissue Valve With Linx™ AC Technology
Read moreTen-Year Outcomes of a Contemporary Supra-annular Porcine Aortic Bioprosthesis in a Medicare Population1

Epic™ Mitral Stented Tissue Valve With Linx™ AC Technology
Read more
Epic Mitral – Ten Year Clinical Outcomes of SMVR in a Medicare Population1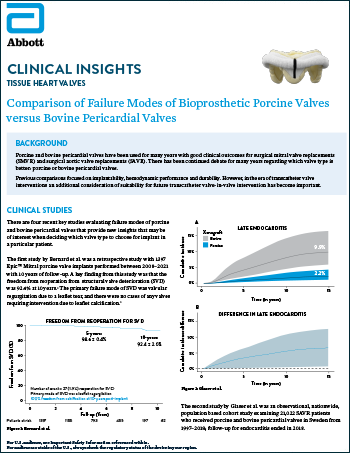
Tissue Heart Valves
Read more
Comparison of Failure Modes of Bioprosthetic Porcine Valves versus Bovine Pericardial Valves
Epic™ Mitral Stented Tissue Valve with Linx™ AC Technology
Read moreEpic Mitral Valve Continues the Biocor Legacy: Strength of Design, Tested Over 35 Years
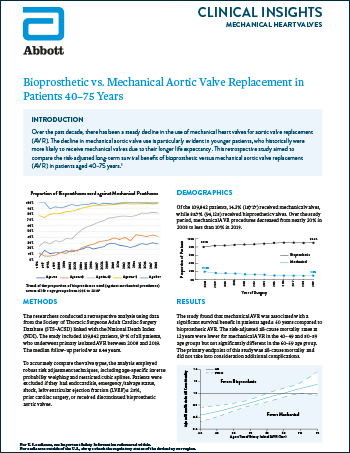
Mechanical Heart Valves
Read moreBioprosthetic vs. Mechanical Aortic Valve Replacement in Patients 40–75 Years
CLINICAL DATA | TEER SOLUTIONS
CLINICAL DATA | SURGICAL VALVE SOLUTIONS
CLINICAL DATA | STRUCTURAL INTERVENTIONS
MAT-2116292 v23.0 | Item approved for U.S. use only.