AMPLATZER™ AMULET™ LAA OCCLUDER CLINICAL DATA
Designed to treat patients with non-valvular atrial fibrillation (AF) who are at risk of ischemic stroke, the Amplatzer Amulet LAA Occluder offers complete closure of the left atrial appendage (LAA) and immediately eliminates the need for oral anticoagulants.1
STROKE RISK REDUCTION, AND FREEDOM FROM ANTICOAGULANTS
Clinical studies have shown that implanting AFib patients with the Amplatzer Amulet occluder is a safe and effective treatment option to reduce their risk of stroke and eliminate the need for oral anticoagulants.
99%3
SUCCESS
RATE
99%1
effective
closure
96%2
free from OAC use after 3 years
67%3
reduction in stroke risk
CLICK THE LINKS BELOW TO LEARN MORE
ABOUT AMULET OCCLUDER DATA
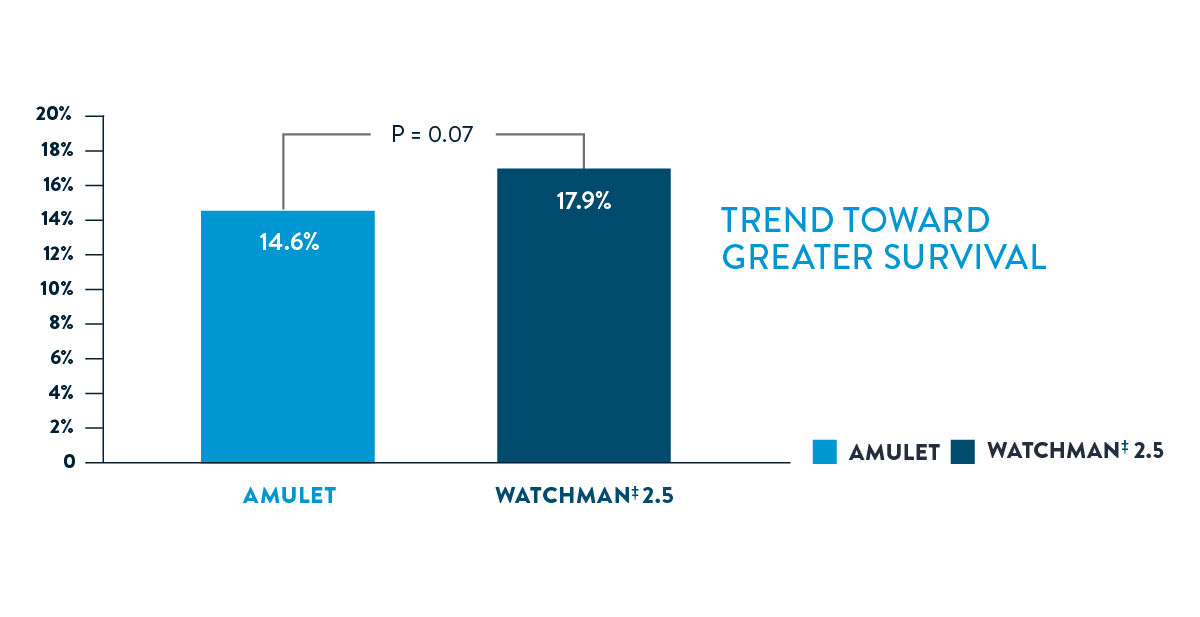
THE AMULET OCCLUDER DEMONSTRATES SUPERIOR CLOSURE, SIGNIFICANTLY HIGHER FREEDOM FROM OACS AND A TREND TOWARD GREATER SURVIVAL
Head-To-Head study demonstrates 63% lower risk of moderate or greater leaks with Amulet occluder4
Amulet occluder patients had significantly higher complete LAA closure rate by TEE compared to Watchman‡ device at both 45 days and 12 months.
‡Indicates a third party trademark, which is property of its respective owner.
- Superior Closure
The Amulet Occluder showed significantly better closure compared to Watchman‡ 2.5 and Watchman‡ flx5,6
AMULET IDE CLOSURE AT 12 MONTHS
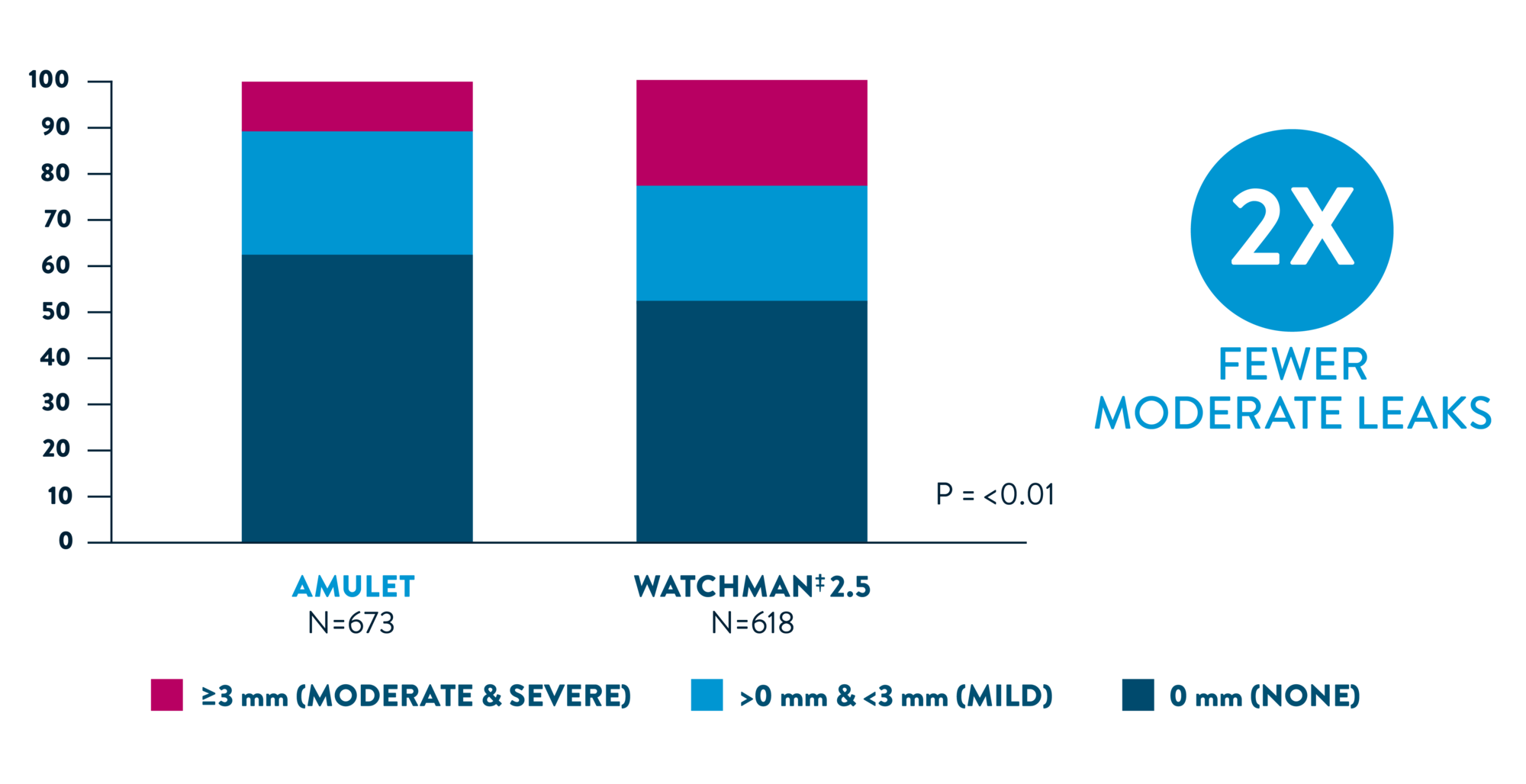
SWISS-APERO COMPLETE CLOSURE AT 45 DAYS BY TEE
SIGNIFICANTLY HIGHER COMPLETE CLOSURE

- Better Outcomes
The Amulet Occluder showed significantly higher freedom from OACS and a trend toward greater survival2
AMULET IDE ORAL ANTICOAGULATION USAGE
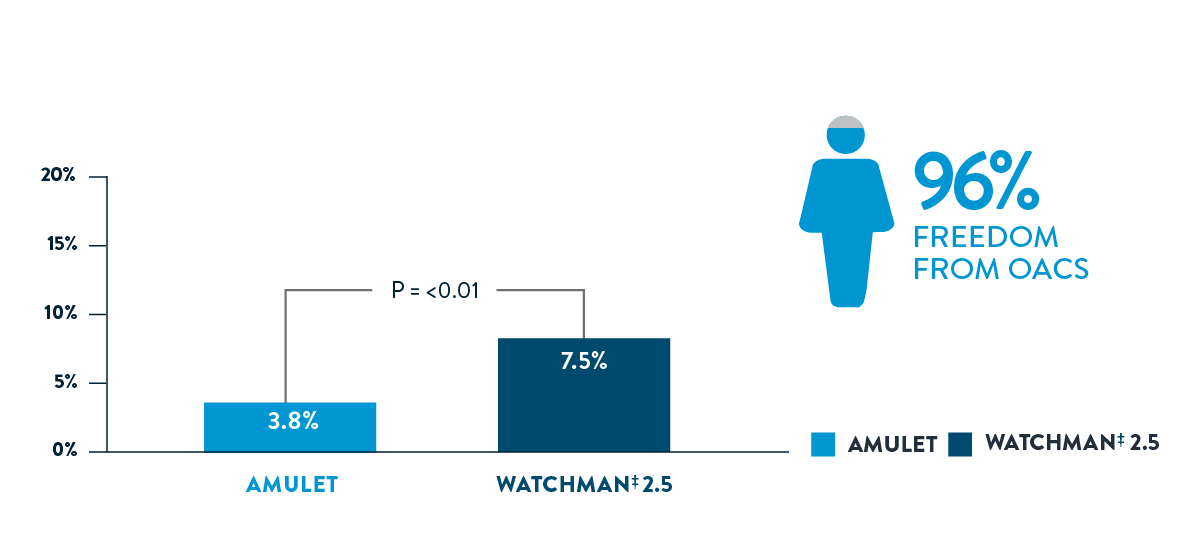
AMULET IDE ALL-CAUSE DEATH AT 3 YEARS
MAT-2212398 v3.0 | Item approved for U.S. use only.