TENDYNE™ TRANSCATHETER MITRAL VALVE REPLACEMENT (TMVR)
The first transcatheter mitral valve replacement system approved in the United States to treat symptomatic severe mitral valve dysfunction associated with severe mitral annular calcification (MAC) in patients who are deemed unsuitable for mitral valve surgery or transcatheter edge-to-edge repair.
DESIGNED FOR MAC. BUILT FOR LIFE.
Tendyne™ TMVR has demonstrated consistent Mitral Regurgitation (MR) elimination,* leading to improved mitral valve function and meaningful gains in quality of life.1

Product & procedural excellence
- Predictable and sustained MR elimination* at 1 year
- Excellent hemodynamics
- Quality of life improvement
- High technical success rate
- Improvement in heart failure symptoms assessed by NYHA classification
*Elimination of moderate-to-severe and severe MR
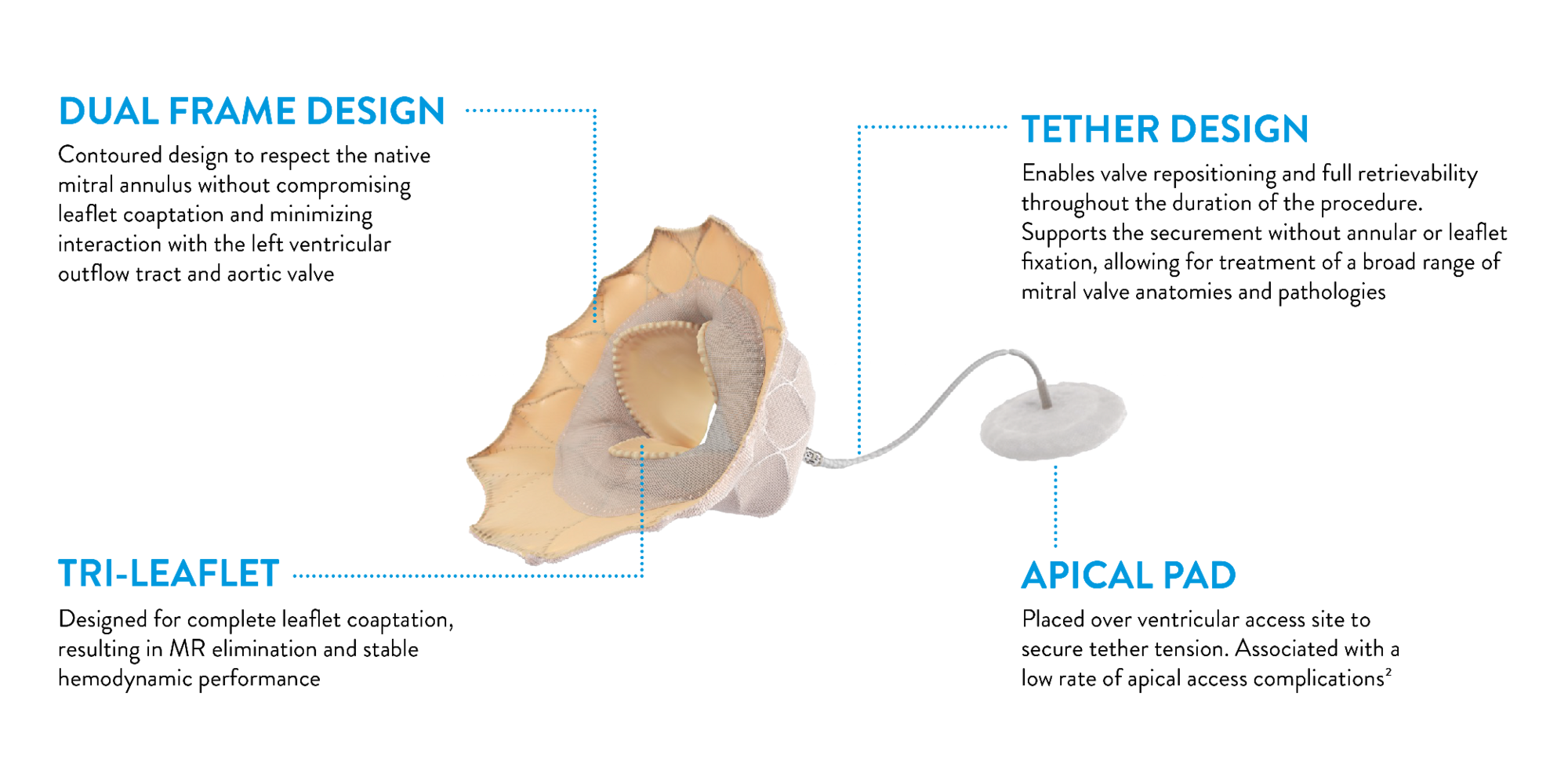
Design
- Transcatheter beating-heart procedure without the need for cardiopulmonary bypass
- Interprocedurally recapturable, repositionable, and retrievable
- Tether design enables securement without annular or leaflet fixation
- Available in five implant sizes to treat patients with varying levels of MAC severity
PATIENTS WHO BENEFIT

Tendyne™ TMVR offers patients with severe mitral valve disease caused by MAC with a new option for safe and effective mitral valve replacement.
FEATURES
Tendyne™ Valve overview
Procedure overview
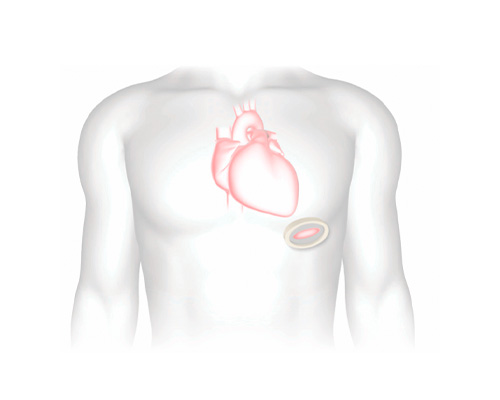
A delivery system positions the Tendyne valve in the heart via an incision in the left side of the chest.
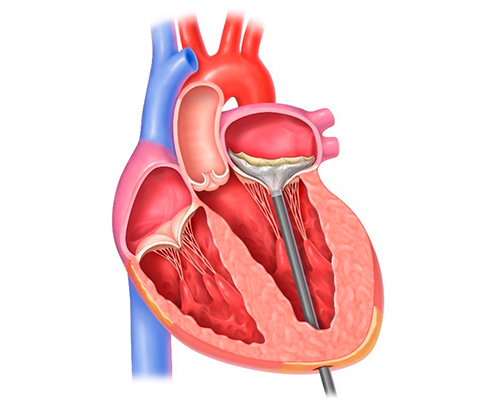
Once the Tendyne valve is optimally positioned within the mitral annulus, the delivery system is removed.
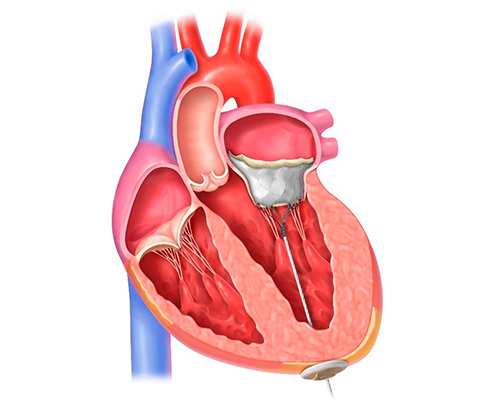
Applying tension to the tether assists in securing the valve, which is attached to an apical pad placed over the access site.
MAT-2512510 v2.0 | Item approved for U.S. use only.