NAVITOR™ TAVI SYSTEM
CLINICAL DATA
Explore the latest TAVI clinical data for Navitor valves: 0% moderate or severe PVL, low mortality and stroke rates, and excellent hemodynamics.
PIONEERING CLINICAL EXCELLENCE
IN HEART VALVE REPLACEMENT
The Navitor TAVI* System demonstrates exceptional clinical trial results in transcatheter aortic valve implantation. Engineered for patients with severe aortic stenosis considered high-risk for traditional surgery, the Navitor TAVI System represents a significant advancement in minimally invasive heart valve replacement.
*TAVI is also referred to as TAVR (Transcatheter Aortic Valve Replacement)
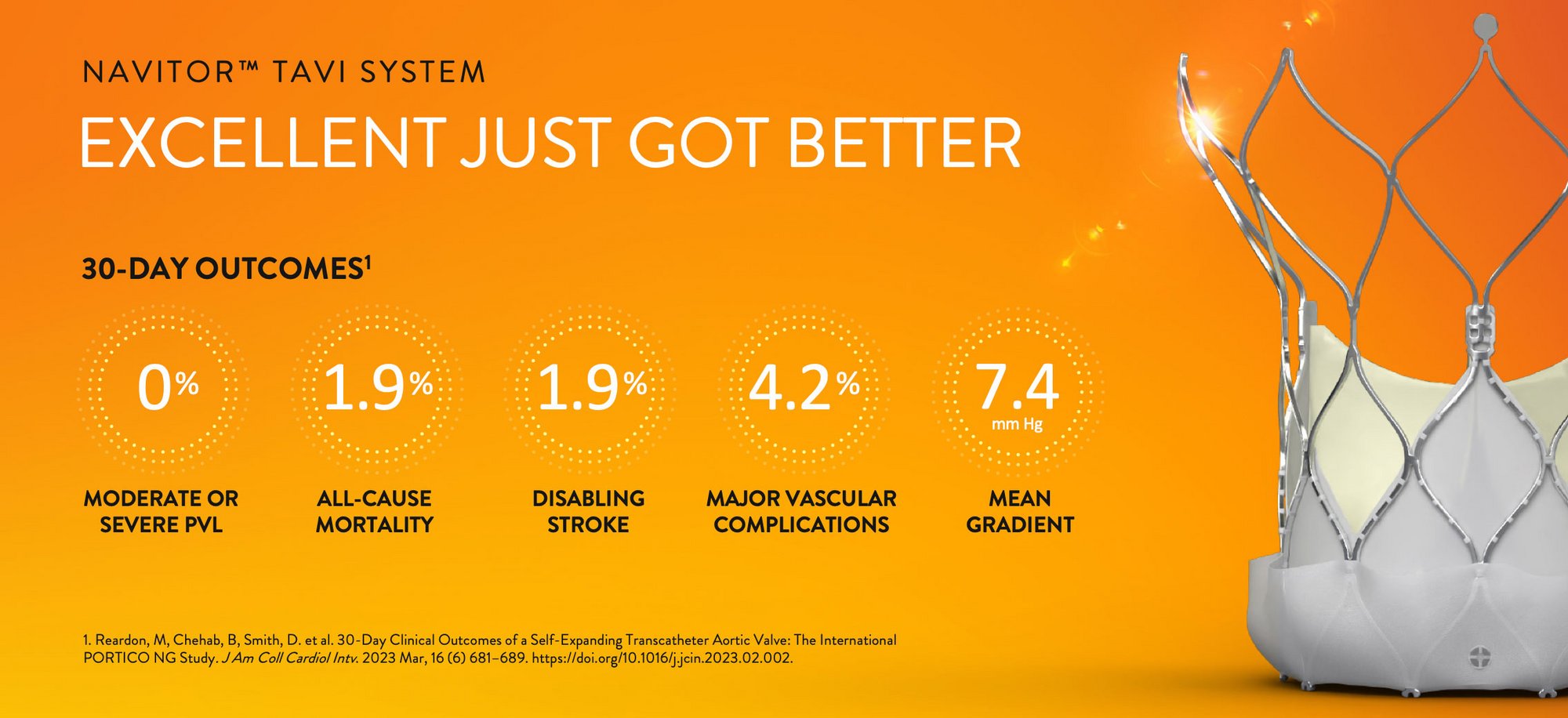
Acute Outcomes for Navitor TAVI
The 30-day clinical outcomes of the Navitor TAVI System highlight its advanced design and its role in providing a safe, effective treatment option for those at high or greater risk for traditional surgery, emphasizing its precision and the minimized risk of complications associated with the procedure.
| 30-DAY | NAVITOR™2 N=260 |
|---|---|
| All-Cause Mortality | 1.9% |
| Disabling Stroke | 1.9% |
| Life-Threatening Bleeding | 3.8% |
| Acute Kidney Injury Stage 2/3 | 1.9% |
| Major Vascular Complications | 4.2%* |
*3.1% access site-related, and 1.2% non-access site-related.
0% Moderate or Severe PVL
| PVL 30-DAY ECHO CORE LAB DATA | NAVITOR™2 N=248 |
|---|---|
| None/Trace | 79.8% |
| Mild | 20.2% |
| Moderate | 0.0% |
| Severe | 0.0% |
Based on number of subjects with data evaluable by the echo core lab.
Excellent Hemodynamics in TAVI With Navitor Valves
The Navitor valves feature a unique cylindrical stent and intra-annular leaflets that open fully to maximize orifice area and achieve single digit mean gradients.
| 30-DAY ECHO CORE LAB DATA | NAVITOR™2
|
|---|---|
| Mean Gradient (mm Hg) | 7.4 (N=249) |
| EOA (cm2) | 2.0 (N=215) |
Based on number of subjects with data evaluable by the echo core lab.
CONNECT WITH YOUR LOCAL REPRESENTATIVE TODAY
MAT-2214676 v4.0 | Item approved for U.S. use only.