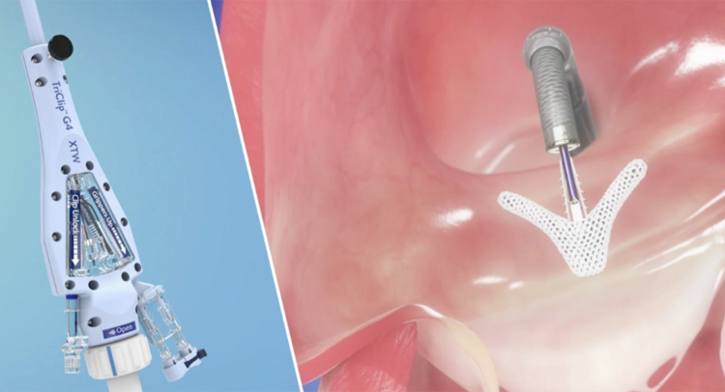
TRICLIP™ TRANSCATHETER TRICUSPID VALVE REPAIR SYSTEM
TriClip™ Transcatheter Edge-to-Edge Repair (TEER) offers a minimally invasive treatment option to improve quality of life (QoL) and functional status in patients with symptomatic severe tricuspid regurgitation, despite optimal medical therapy, who are at intermediate or greater risk for surgery.1
INSPIRED BY PATIENTS.
MADE POSSIBLE BY YOU.
Choose Safety. Choose Repair.
TriClip Transcatheter Edge-to-Edge device is the only TEER device intentionally designed for the tricuspid valve, with stable navigation and precise delivery2,14 to safely maximize tricuspid regurgitation (TR) reduction and achieve sustained clinically significant improvement in the quality of life of patients with TR.3,4
- Significant TR reduction3,4
- Exceptional safety profile4,5
- Life-changing improvements in health related quality of life3-5
- Proven in randomized and real-world studies.3,4
PATIENTS WHO BENEFIT
A state-of-the-art treatment option for patients at intermediate
or greater risk for surgery
TriClip TEER is a low-risk,5 non-surgical treatment option for improving quality of life and functional status in patients with symptomatic severe tricuspid regurgitation, despite optimal medical therapy, who are at intermediate or greater risk for surgery and in whom transcatheter edge-to-edge valve repair is clinically appropriate and is expected to reduce tricuspid regurgitation severity to moderate or less, as determined by a multidisciplinary heart team.1
- Transcatheter beating heart procedure
- no cardiopulmonary bypass - Allows for real-time positioning and
repositioning to optimize TR reduction - Femoral venous access
- Can be used in a standard cath lab or hybrid room
- No pre-procedural CT required
- Fast recovery times; many patients go home
the next day4
- Patients with severe tricuspid regurgitation have significantly impaired quality of life12
- Shortness of breath
- Peripheral edema
- Ascites
- Fatigue
- Declining exercise capacity
- Current treatment options have limitations
Medical therapy for TR has little impact on TR reduction5
66%
of patients with severe FTR die within 5 years of medical management6
Most patients receive medical management until right heart failure or end-organ dysfunction appears.7 - Surgery is high risk and often denied8,9
Surgery for TR is seldom performed. Factors prohibiting surgery include:
- High operative risk (6-16% in-hospital mortality)
- Multiple comorbidities
- Advanced age
- Lack of effectiveness
Long-term survival estimates for all patients undergoing
surgical tricuspid valve replacement or repair10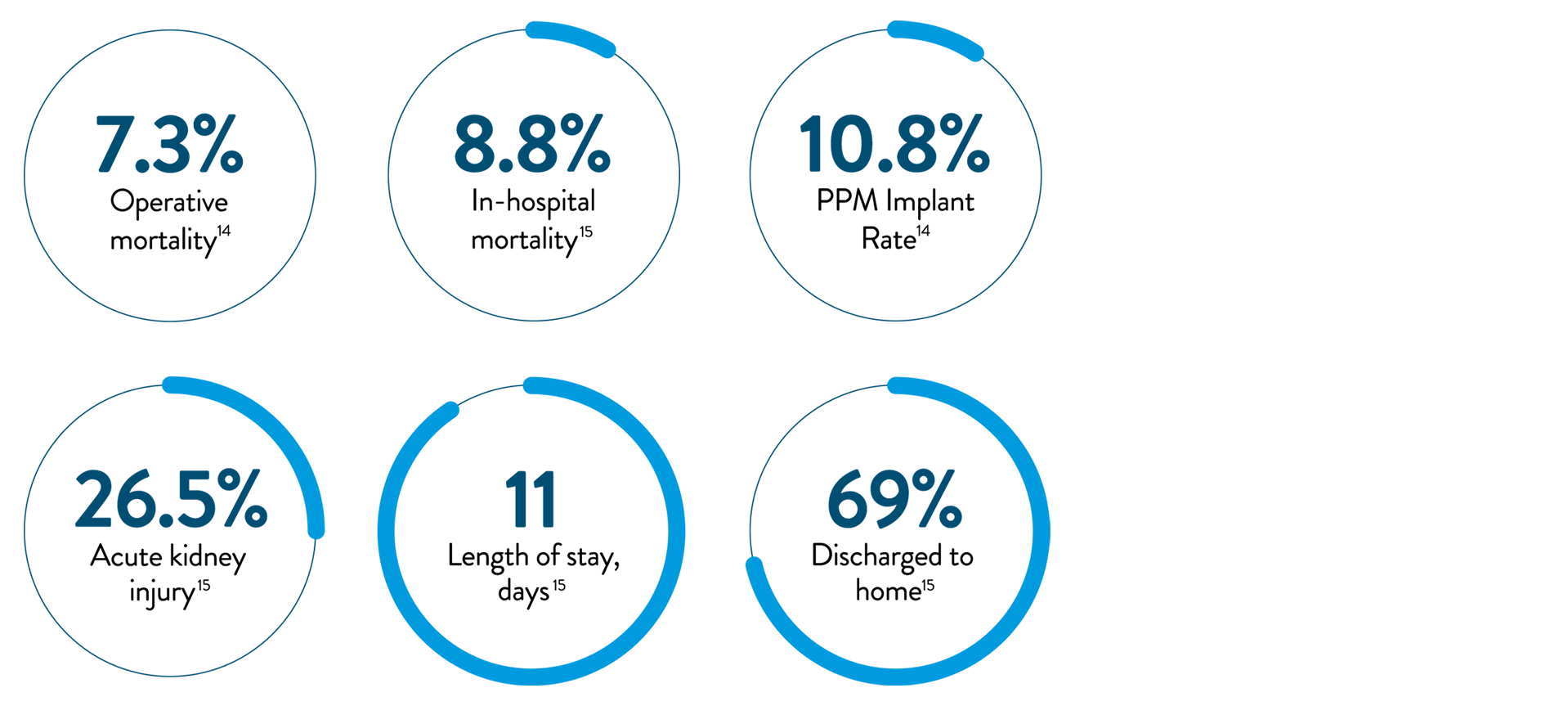
FEATURES

EXCEPTIONAL SAFETY4,5

MAXIMIZED EFFECTIVENESS4,5

LIFE-CHANGING IMPACT3-5
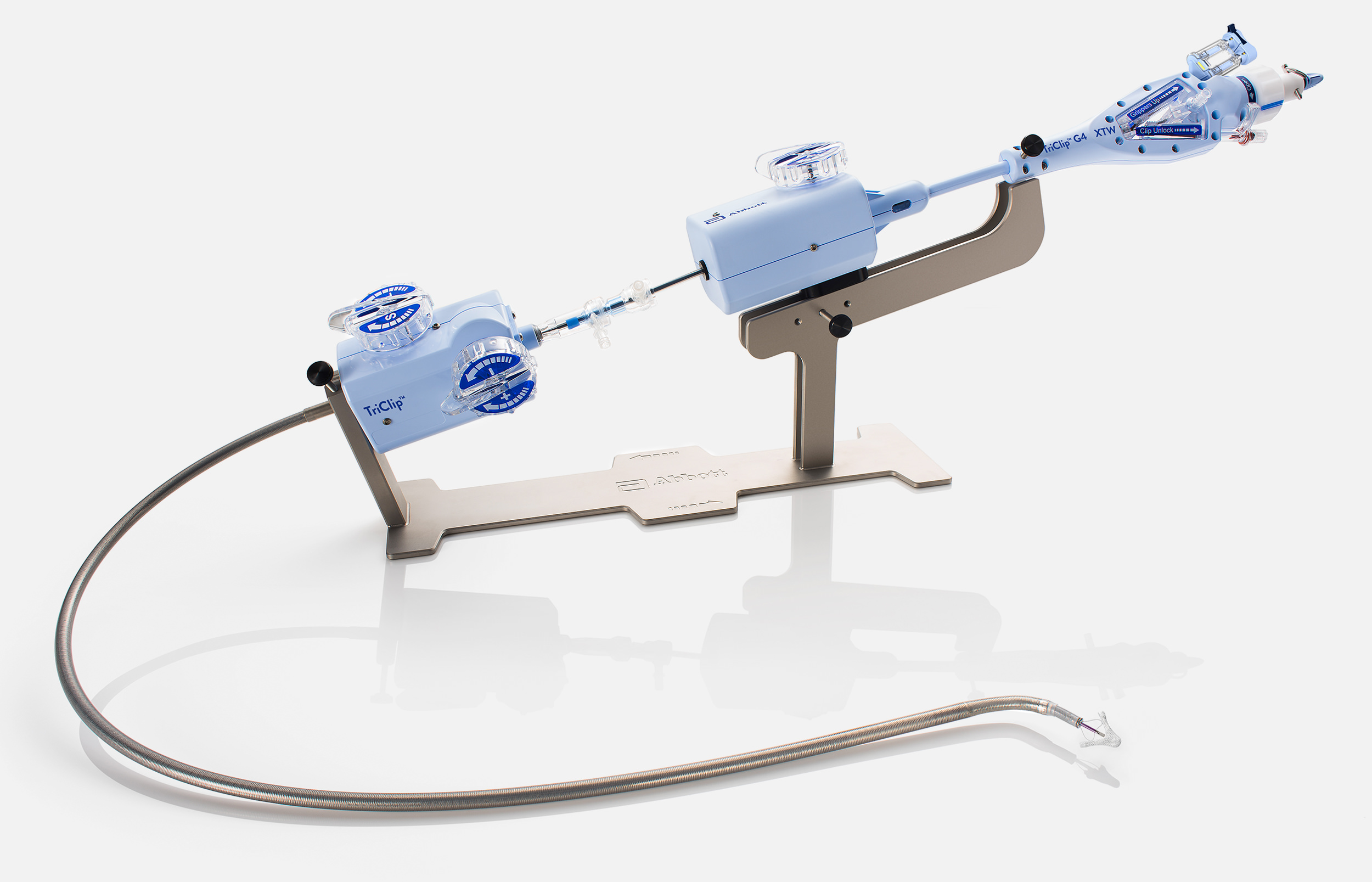
The TriClip™ G4 Transcatheter Edge-to-Edge Repair System empowers you with stable navigation and precise delivery2
for complex conditions.
F/E Knob
S/L Knob
Steerable Guide Catheter
+/- Knob
Distal Curve
TriclipTM G4 Delivery System
Controlled Gripper Actuation
Actuator Knob
Flexes and extends delivery catheter to steer down to the valve plane
Enables movement in septal or lateral direction
- Designed for the tricuspid anatomy2,14,15
- Provides adequate height over the valve
- Maintains coaxial position during steering and positioning
- Allows sweeping away from the septum to optimize delivery catheter position while maintaining perpendicularity to the valve plane
Straightens or curves the guide to add or subtract height above the valve
Designed for direct access to the tricuspid valve2,14,15
- Provides stability and precision during steering and positioning
- Multiaxis steering to enable navigation across all lines of coaptation
Confirm and optimize leaflet grasping
Implant and gripper line detachment
- Provides stability and precision during steering and positioning
- Multiaxis steering to enable navigation across all lines of coaptation
- Flexes and extends delivery catheter to steer down to the valve plane
- Enables movement in septal or lateral direction
- Straightens and curves guide for height adjustment above the valve
Designed for the tricuspid anatomy2,14,15
- Provides adequate height over the valve
- Maintains coaxial position during steering and positioning
- Allows sweeping away from the septum to optimize delivery catheter position while maintaining perpendicularity to the valve plane
- Designed for direct access to the tricuspid valve2,14,15
- Confirm and optimize leaflet grasping
- Implant and gripper line detachment
Test(s) performed by and data on file at Abbott.
- Building on a legacy of unmatched TEER expertise13
TriClip™ implants use the same proven leaflet repair technology as our MitraClip™ Transcatheter Edge-to-Edge Repair (TEER) implants. TriClip TEER is the clip-based technology physicians know and trust, with a delivery system uniquely designed for the tricuspid valve.
TriClip implant features1:
- Elgiloy® implants with nitinol grippers
- Polyester cover designed to promote tissue growth
- Magnetic resonance conditional to 3 Tesla†
†Static magnetic field of 1.5 T or 3 T; maximum spatial gradient in static field of 4000 gauss/cm or less; maximum whole-body averaged specific absorption rate (SAR) of 2 W/kg for 15 minutes of scanning.
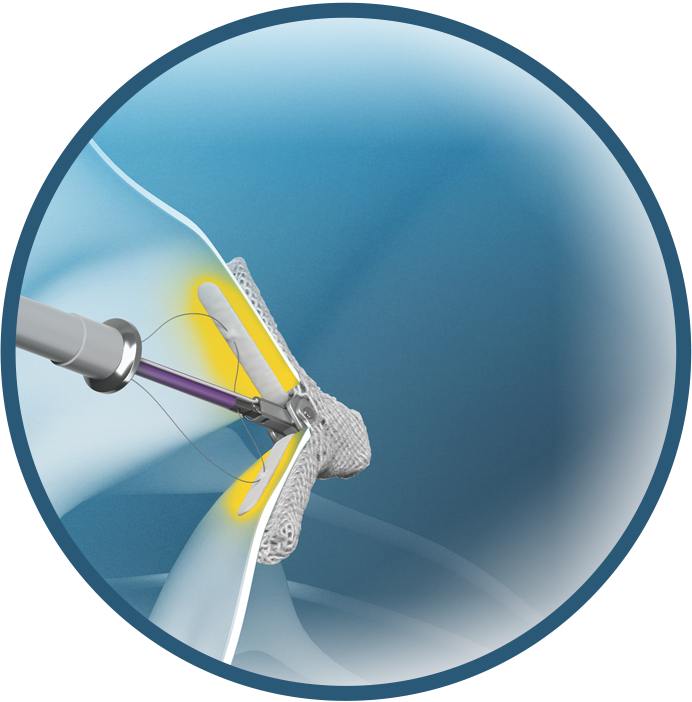
- Optimized leaflet capture
Optimized leaflet capture for consistent TR reduction with the TriClip G4 TEER System
- Wide grasp opening allows full leaflet insertion1
- Designed to distribute retention forces along the entire inserted leaflet length11
- Four or six rows of short frictional elements designed to provide atraumatic leaflet engagement11
- Broad range of sizes for tailored treatment
Four implant sizes are available for patient-specific therapy with the TriClip G4 TEER System.1

CLINICAL DATA
See the latest clinical data for the Triclip™ Transcatheter Tricuspid Valve Repair System.
MAT-2401619 v6.0 | Item approved for U.S. use only.