IF YOU HAVE PRIMARY MITRAL REGURGITATION, YOU MAY BE ELIGIBLE FOR THE REPAIR MR TRIAL
IF YOU HAVE PRIMARY MITRAL REGURGITATION, YOU MAY BE ELIGIBLE FOR THE REPAIR MR TRIAL

The REPAIR MR Clinical Trial is studying the MitraClip™ Transcatheter Mitral Valve Repair system in patients with severe primary mitral regurgitation who are at moderate surgical risk and whose mitral valve has been assessed as suitable for correction by mitral valve repair surgery.
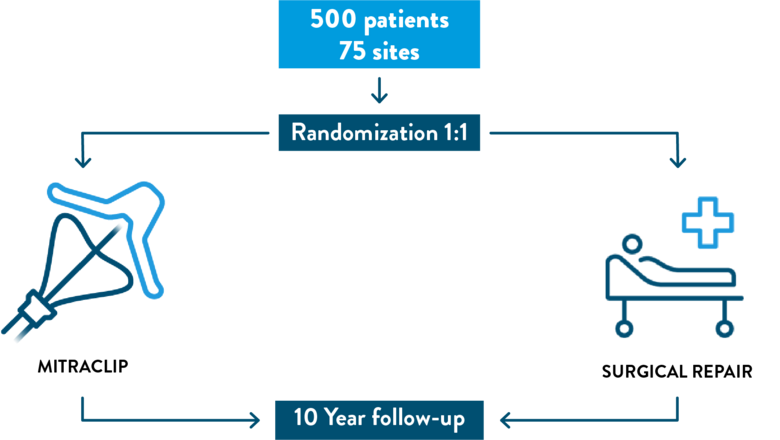
Approximately 500 subjects will be studied at up to 75 medical centers in the United States, Canada, and Europe. A qualified team of doctors will monitor the patients included in the REPAIR MR Clinical Trial.
Study participants will play an important role in helping doctors evaluate transcatheter mitral valve repair as an option for patients with primary mitral regurgitation who are candidates for surgery.
NCT04198870 ClinicalTrials.gov
Find a clinical trial site near me
WHAT IS
MITRAL REGURGITATION (MR)?
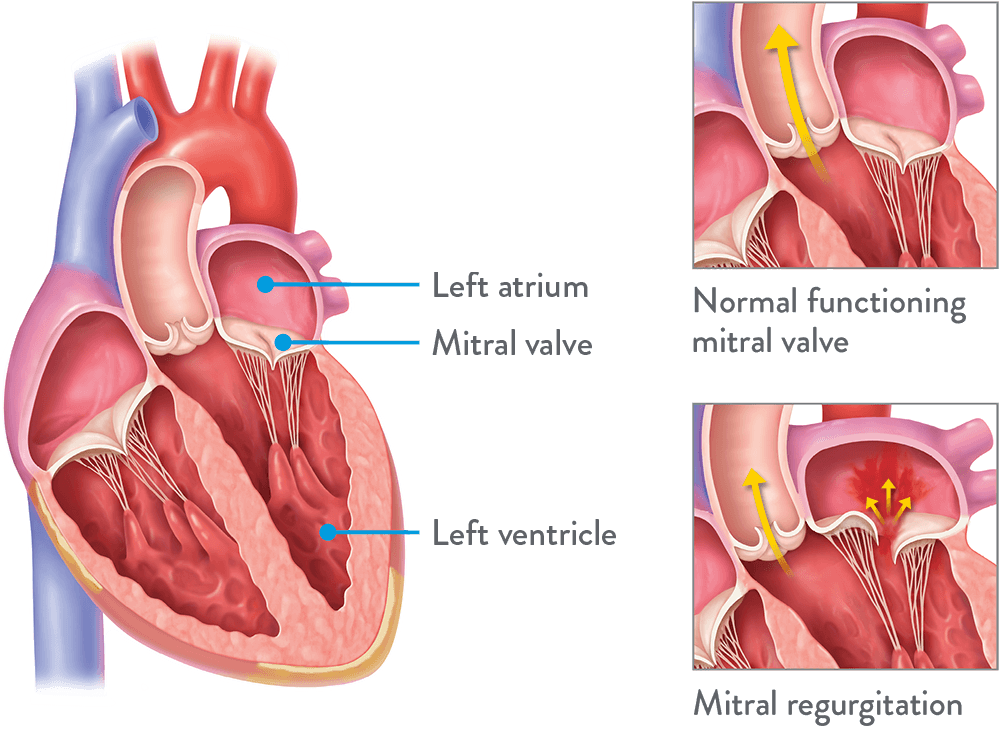
The mitral valve is a valve in your heart that opens to let blood flow forward and closes to prevent backflow of blood.
Mitral regurgitation (MR) happens when there is leaking because your mitral valve doesn’t close tightly enough.
This abnormality allows blood to flow back in your heart, which can cause the heart to enlarge, weaken the heart muscles, and manifest as other systems affecting your quality of life.
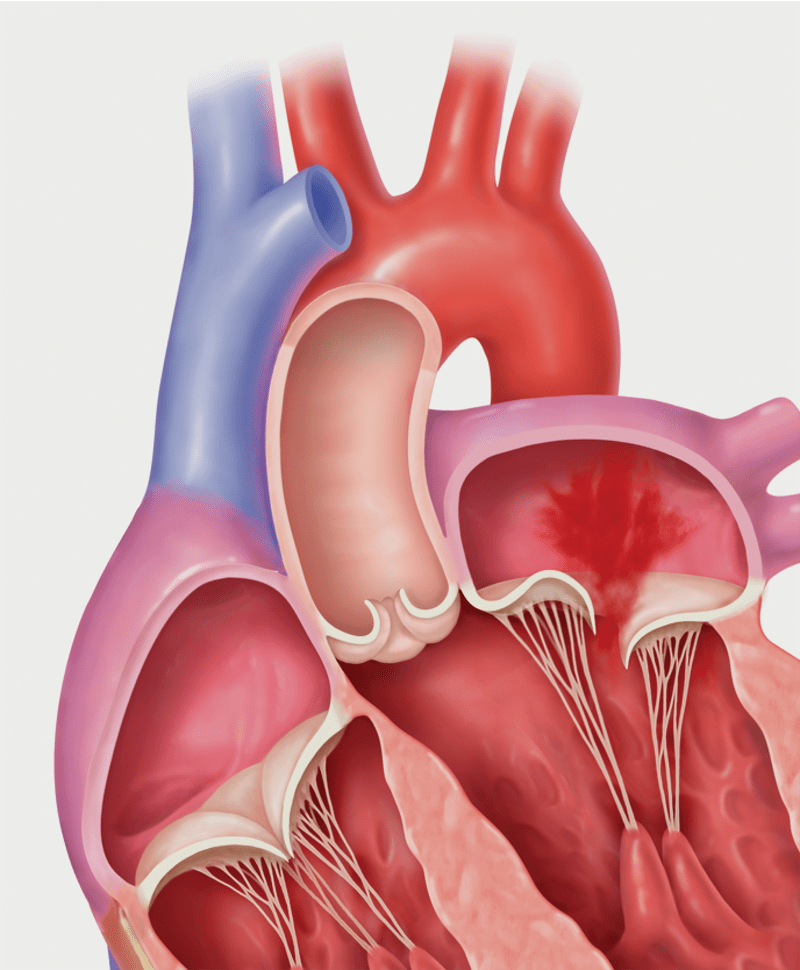
- Two types of Mitral Regurgitation: Primary vs. Secondary MR
Primary MR, also called degenerative MR, is caused by damage to the mitral valve with prolapse or flail of the leaflets. It can be related to age, a birth defect, or underlying heart disease. Secondary MR, also called functional MR, is caused by enlargement of the heart due to heart attack or heart failure.
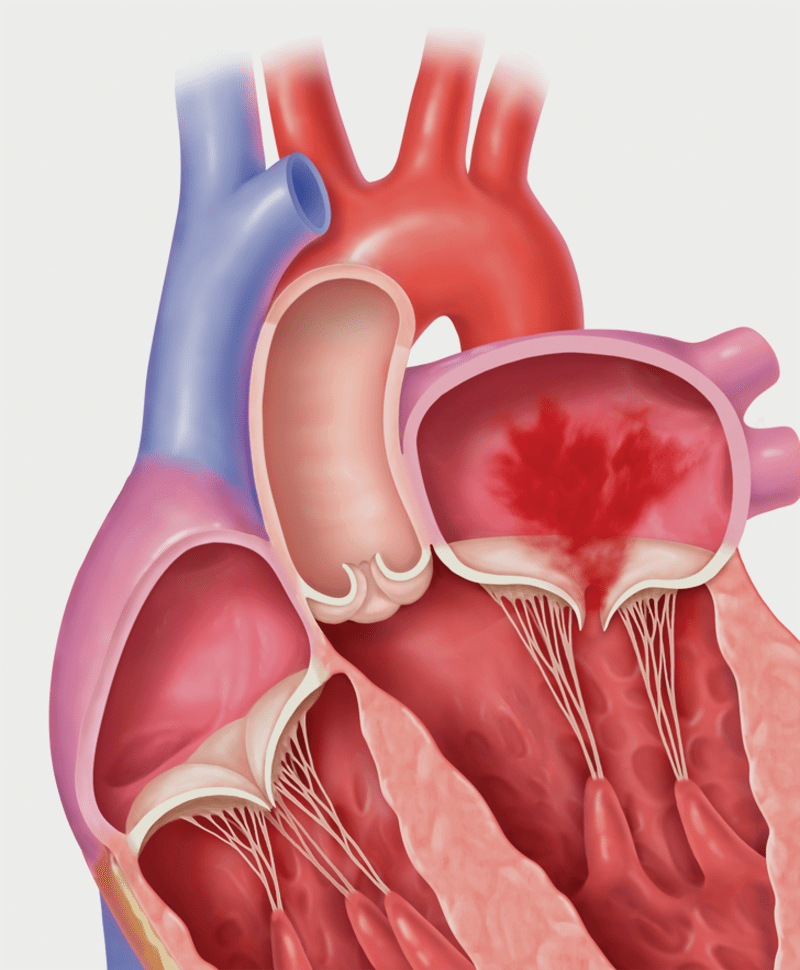
Primary MR (Degenerative MR): Prolapse
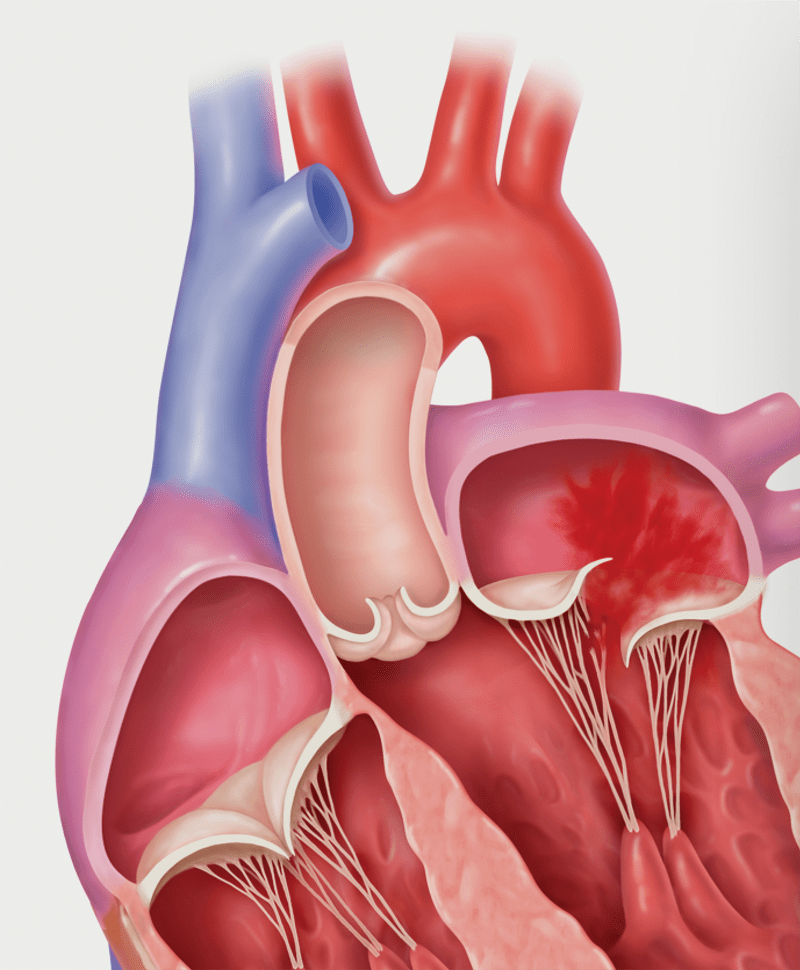
Primary MR (Degenerative MR): Flail
Secondary MR (Functional MR)
How is
mitral regurgitation treated?
GUIDELINE-DIRECTED
MEDICAL THERAPY (GDMT)
Medication may be prescribed to manage symptoms of MR, however, it does not eliminate the root cause.
MITRAL VALVE
SURGERY
Open-heart surgery may be an option to have the mitral valve repaired or replaced.
TRANSCATHETER
MITRAL VALVE REPAIR
In select patients, a minimally invasive transcatheter beating heart procedure is conducted to repair the mitral valve.
What is
Transcatheter Mitral Valve Repair?
Transcatheter Mitral Valve Repair of the Mitral Valve is a minimally invasive treatment option for patients who are deemed appropriate for this procedure. The procedure is done using MitraClip™ Transcatheter Mitral Valve Repair.
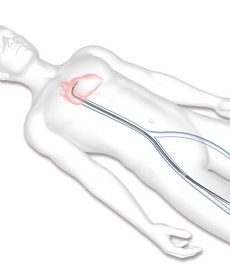
STEP 1
A guide catheter (a thin tube) will be inserted through a vein from a small cut in your upper leg to reach your heart
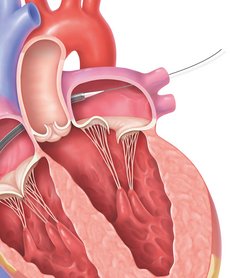
STEP 2
The MitraClip implant, a small clip on the end of a second delivery catheter, will be guided to your mitral valve though the guide catheter.
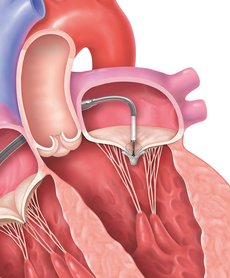
STEP 3
The implant will be positioned and the clip will grasp the mitral valve leaflets to close the leaking jet in your mitral valve and reduce MR.
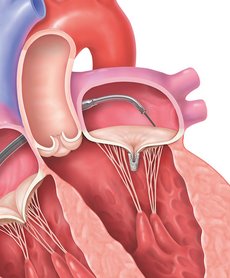
STEP 4
Once the implant is in place, it will be separated from the clip delivery system.
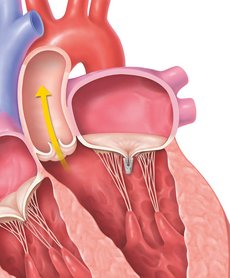
STEP 5
The implanted clip will become a permanent part of your heart, allowing your mitral valve to close more tightly and reduce the backward flow of blood.
ARE YOU A CANDIDATE FOR
THE REPAIR MR TRIAL?
What is involved if I choose to participate?
You will be screened to make sure you are a good candidate for the study. Your screening may include:
- A physical examination and update to medications
- An echocardiogram (ECHO) to determine if you have the appropriate valve anatomy
- A review of your medical history
- Blood tests
What happens if I pass the screening process?
- You will be assessed in-person at the treatment center and then registered in the study.
- You will be assigned to one of two treatment groups: mitral valve surgery or transcatheter mitral valve repair with the MitraClip System.
- You will undergo your assigned treatment.
- You will have in person or phone call follow-up exams with healthcare providers at 30-days and annually for up to 10 years.
- You will have the option to have some of your in-person follow-ups in the comfort of your own home
The REPAIR MR
patient journey
FREQUENTLY
ASKED QUESTIONS
- What will happen if I decide I am interested to take part in this trial?
This research study includes a screening/qualification phase to determine if you are a good candidate for the study. Your trial doctor or other study personnel will ask you medically related questions. Additional tests will be conducted to determine if you qualify for the study. These evaluations will include a physical exam (i.e. height, weight, blood pressure, heart rhythm) and blood test (needle stick to a vein in the arm to withdraw blood). Once all tests have been completed, your trial doctor will decide if you qualify to take part in the REPAIR MR study. If you do not qualify for the study, your participation will end.
If your trial doctor determines that you qualify, and you decide to take part in this research study, your involvement will last approximately 10 years. You will be asked to return to the clinic approximately 10 times. You are expected to complete all required follow-up visits.
- Do I have a choice in what mitral regurgitation treatment I receive?
If qualified for the study, you will not have a choice in what treatment you receive as REPAIR MR is a controlled randomized study.
Randomization is the process by which you will be randomly (by chance) assigned to either of two groups (control group: surgical mitral valve repair, or treatment group: Transcatheter Repair using MitraClip). Randomization is like a coin toss in deciding which subject group you will be part of. A computer program will decide which group you will be in and you will have equal chance of being assigned to the control group or to the treatment group.
Due to the nature of the treatment, you as well as your doctor who performs the MitraClip procedure or the mitral valve surgery will be aware of the treatment assignment. Irrespective of the group you are assigned, you will return for visits with the trial doctor at the same schedule and will be followed equally closely by the trial doctor.
- What happens if I am randomized to the control group?
If you are assigned to the control group, you will undergo a surgical mitral valve repair, by accessing the heart through an incision in the chest. This is the current standard of care for the treatment of primary mitral regurgitation.
- What happens if I am randomized to the treatment group?
If you are assigned to the treatment group, you will undergo a transcatheter procedure to repair your mitral valve using the MitraClip System.
- Where can I learn more about patients who have been treated with MitraClip Transcatheter Mitral Valve Repair?
You can learn more about the MitraClip Transcatheter Mitral Valve Repair at MitraClip - Mitral Regurgitation Treatment | Patient Site
- What other choices do I have?
You will receive available treatment for mitral regurgitation whether or not you take part in this trial. If you choose not to take part in this trial, your doctor will treat you according to standard medical care. Other options may include continuing to take medication to treat your heart condition. Your doctor will explain in more detail the various alternative procedures or therapies available to you and the risks and benefits of these alternatives based on the anatomy of your heart. Your future care and your relationship with your doctor will not be affected if you decide not to be in this trial.
MitraClip™ Transcatheter Mitral Valve Repair
MitraClip is an FDA approved device being studied in a new group of patients within the REPAIR MR Trial. The use of MitraClip in this trial is considered investigational in the US. In the REPAIR MR patient population, the device is limited by US law to investigational use only and not for sale.
- Importance of clinical research and the REPAIR MR Trial
- The REPAIR MR Trial is evaluating MitraClip in patients with primary mitral regurgitation who are at moderate surgical risk and whose mitral valve has been suitable for correction by MV repair surgery. The results of this trial may expand availability of the minimally invasive transcatheter mitral valve repair system to a new group of patients who are candidates for surgery.
- This trial allows doctors to compare treatments (surgery and MitraClip) for effectiveness, safety, and cost to the healthcare system.
- May help future patients with similar health conditions receive the best treatment.
- The MitraClip is FDA approved, but currently only for patients at high/prohibitive surgical risk. This study may allow patients to receive treatments that may not otherwise be available to them.
MAT-2300696 v2.0 | Item approved for U.S. use only.