CEPHEA MITRAL VALVE DISEASE REGISTRY
IF YOU HAVE SYMPTOMATIC MITRAL VALVE DISEASE, YOU MAY BE ELIGIBLE.
Mitral Valve Disease
A conversation about a new clinical registry

You may be eligible to take part in a new clinical Registry to help doctors understand more about mitral valve disease by collecting experience from patients with the disease. This approved clinical Registry will gather information about when patients were diagnosed, medical therapy/treatment received and any challenges of living with the disease. The Registry results will provide insight to guide future treatment, with the goal of improving patient care.
Approximately 1000 patients will be enrolled in the Registry from 100 medical centers around the world.
A qualified team of doctors and other healthcare providers will monitor patients throughout the Registry. In subsequent stages of the study, eligible patients may undergo treatment with the Cephea TMVR device.
Find a clinical trial site near me
Visit ClinicalTrials.gov to learn more about the trial and see participating clinics.
WHAT IS
MITRAL VALVE DISEASE?
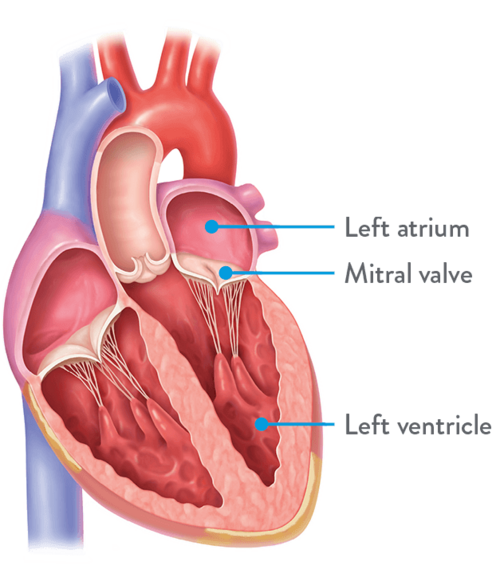
The mitral valve is on the left side of the heart between the upper chamber (atrium) and the lower chamber (ventricle). The valve plays an important role in controlling blood flow between the chambers of the heart by opening and closing.
- Mitral valve disease is a heart condition that includes mitral regurgitation, mitral stenosis and mixed mitral disease.
- Mitral regurgitation is the backwards flow of blood back into the upper chamber instead of forward flow into the lower chamber due to leakage when the heart pumps.
- Mitral stenosis causes reduced blood flow across the valve when the opening is narrowed.
- Mixed mitral valve disease is a condition when both regurgitation and stenosis occur at the same time.
What are
treatment considerations?
Based on the type of mitral valve disease and its severity, your doctor will recommend the best treatment for you. Treatment options include:

Medications

Open-heart surgery

Minimally-invasive transcatheter valve repair
A new treatment option that is currently in the clinical research phase is transcatheter mitral valve replacement (TMVR), which involves a minimally-invasive procedure to implant a new mitral valve. TMVR is a promising alternative for patients with complex mitral valve disease who aren’t good candidates for the current options. This Registry will study patients who are being considered for TMVR treatment using the Cephea System.
What is the Registry and
why should I consider participating?
A Registry is a clinical research study asking volunteers to help doctors answer specific health questions. By taking part in this study, you partner with healthcare providers to help others better understand what treatments people with mitral valve disease receive. This is important as TMVR is still a new treatment option for mitral valve disease, with doctors and researchers needing to better understand the patients that are good candidates for TMVR and how their health changes over time. The healthcare team are experts in helping patients with mitral valve disease and can offer support throughout your health journey. It can be reassuring to know you are not alone.
By participating in the Registry you are helping others:
- learn more about treatment options for patients with mitral valve disease
- develop new therapies, and
- improve patient care
Declining to participate in a Registry will not impact any treatment choices you make with your doctor.
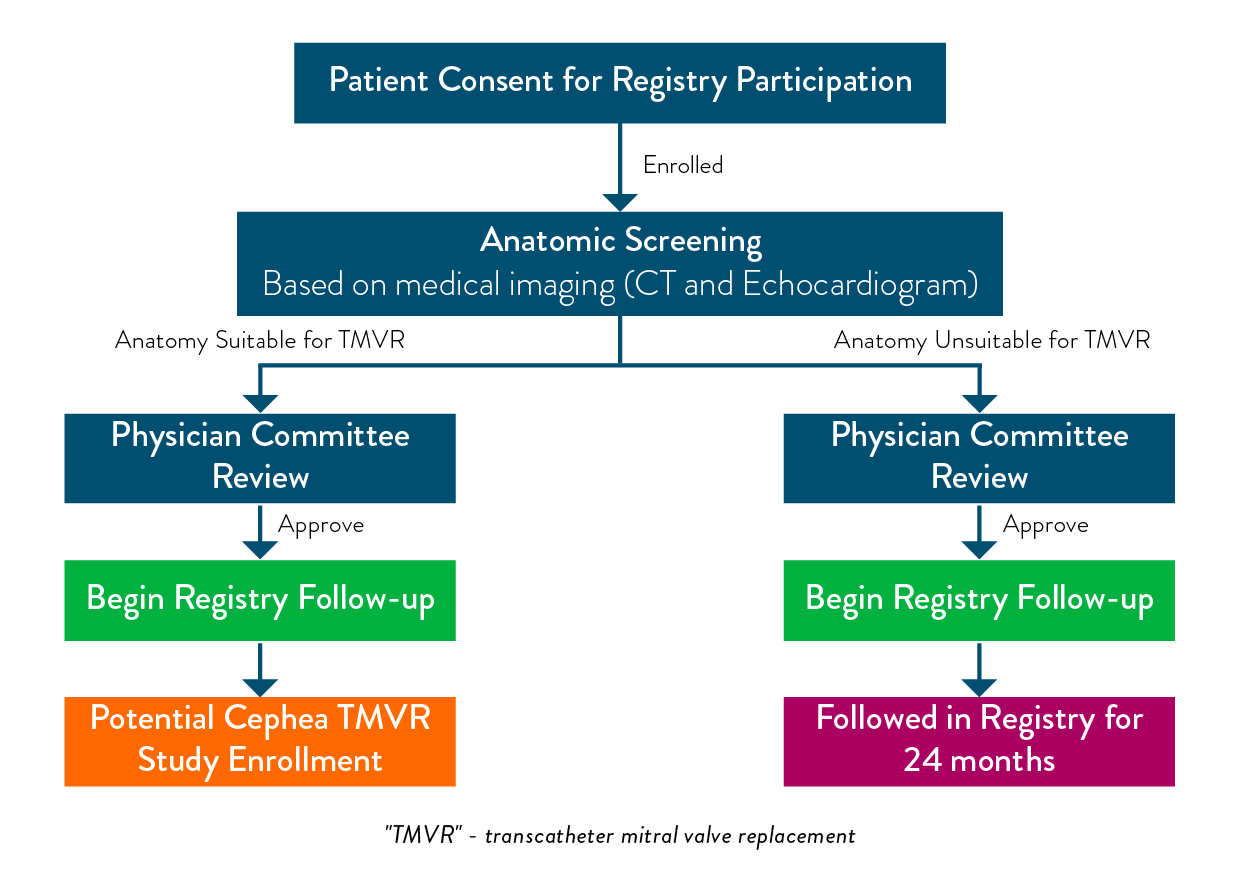
What is involved
with participating in the Registry?
MAT-2511915 v1.0 | Item approved for U.S. use only.
*CAUTION: investigational device. Limited by federal (U.S.) Law to investigational use only.