Cephea Early
Feasibility Study
IF YOU HAVE SYMPTOMATIC MITRAL VALVE DISEASE, YOU MAY BE ELIGIBLE.
Cephea Early Feasibility Study
A clinical trial for patients with symptomatic mitral valve disease

The Cephea* Early Feasibility Study (EFS) is a clinical trial evaluating the preliminary safety and effectiveness of the Cephea Mitral Valve System in patients with mitral valve disease resulting in mitral regurgitation in whom transcatheter therapy is deemed more appropriate than open heart surgery.
Approximately 50 subjects will be studied at up to 20 medical centers in the US, Europe, and Canada. A qualified team of doctors will monitor the patients included in the Cephea Early Feasibility Study.
Study participants will play an important role in helping doctors evaluate transcatheter mitral valve replacement with a less invasive access to the mitral valve for patients who are candidates for transcatheter treatment options.
Find a clinical trial site near me
Visit ClinicalTrials.gov to learn more about the trial and see participating clinics.
WHAT IS
MITRAL REGURGITATION (MR)?
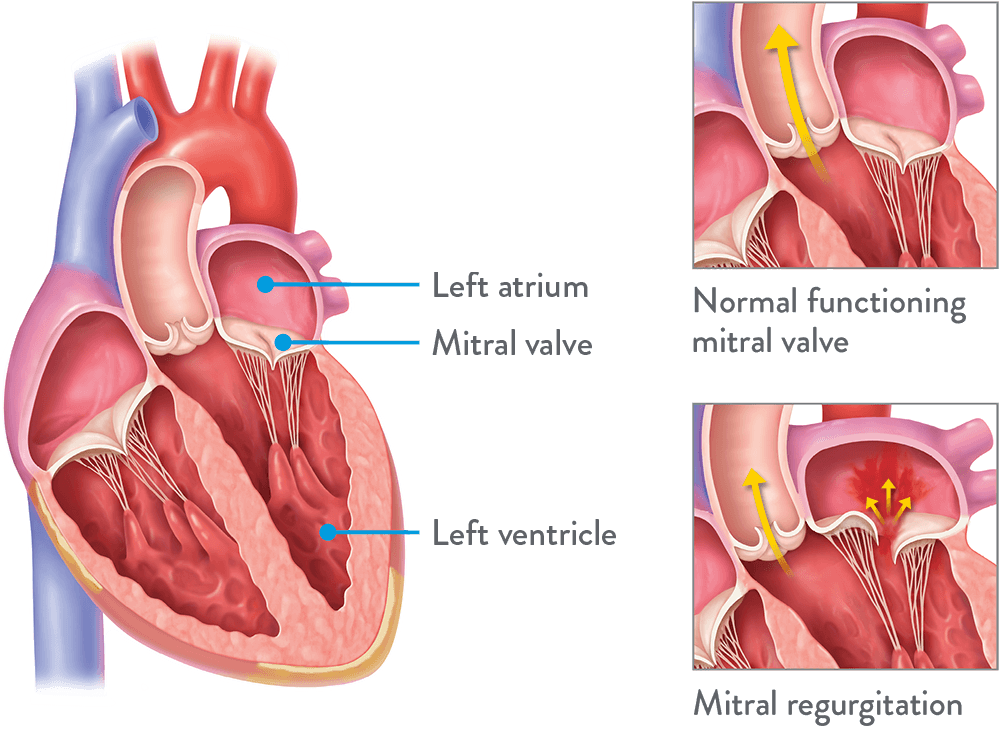
The mitral valve is a valve in your heart that opens to let blood flow forward and closes to prevent backflow of blood.
Mitral regurgitation happens when there is leaking because your mitral valve doesn’t close tightly enough.
This abnormality allows blood to flow back in your heart, which can cause the heart to enlarge, weaken the heart muscles, and can cause symptoms affecting your quality of life.
How is
mitral regurgitation treated?

GUIDELINE-DIRECTED
MEDICAL THERAPY
Medication may be prescribed to manage symptoms of mitral regurgitation, however, it does not eliminate the root case, and therefore a surgical or transcatheter procedure may be offered as additional treatment option.
SURGICAL MITRAL VALVE REPAIR
OR REPLACEMENT
Open-heart surgery may be an option to have the mitral valve repaired or replaced.
TRANSCATHETER MITRAL VALVE REPAIR
OR REPLACEMENT
In select patients, a minimally invasive transcatheter beating heart procedure is conducted to repair the mitral valve.
What is
Transcatheter Mitral Valve Replacement?
Transcatheter mitral valve replacement is a less-invasive treatment option for patients who are deemed appropriate for this procedure. The procedure is done using the Abbott Cephea Mitral Valve Replacement system.
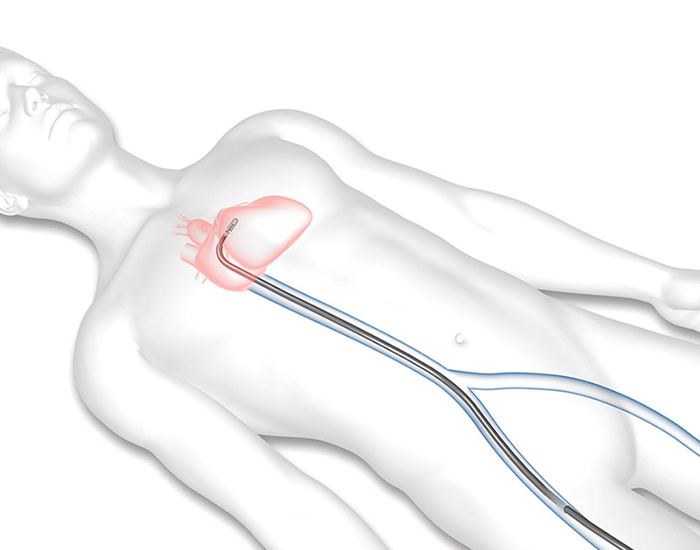
STEP 1
A delivery system will be inserted through your right femoral vein from a small cut in your upper leg to reach your heart.
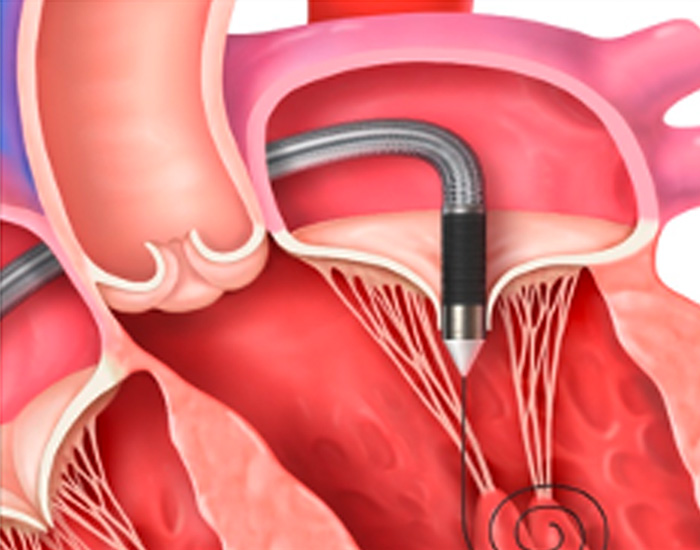
STEP 2
The Cephea implant, which is attached to the end of the delivery system, will be guided to your mitral valve through the catheter.
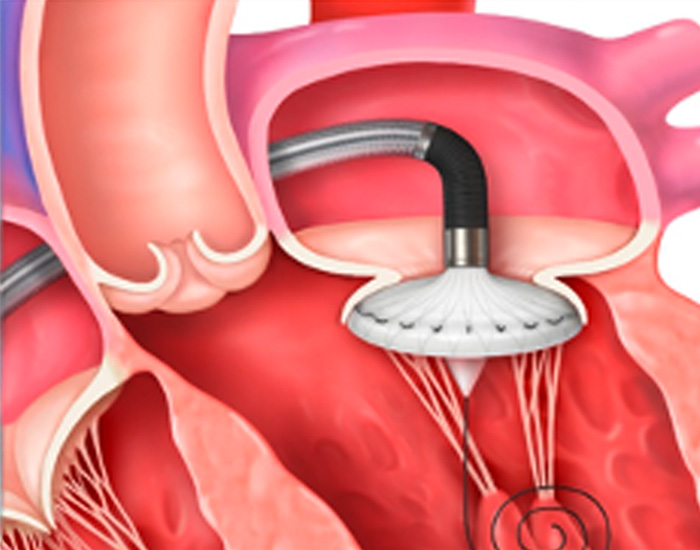
STEP 3
The implant will be positioned into place and deployed through the delivery system. Your physician will monitor the placement of your new valve with imaging technology.

STEP 4
Once the Cephea valve is fully in place, the delivery system will be withdrawn. Your new valve will be a permanent part of your heart and will improve your heart’s function by reducing the backward flow of blood.
ARE YOU A CANDIDATE FOR
THE CEPHEA EARLY FEASIBILITY STUDY?
Who can participate in the CEPHEA EARLY FEASIBILITY STUDY?
Both men and women can participate. You may qualify if:
You have been diagnosed with mitral valve disease (including mitral regurgitation, mitral stenosis or mixed mitral valve disease) and experiencing symptoms due to your valve disease.
What is involved if I choose to participate?
Before you are enrolled, you will be screened to make sure you are a good candidate for the study. Your screening may include:
- A physical examination and update to medications
- An echocardiogram (ECHO) and computed tomography (CT) to determine if your anatomy is appropriate for the Cephea valve
- A review of your medical history
- Blood tests
What happens if I pass the screening process?
- You will be assessed in-person at the treatment center and then registered in the study.
- You will receive treatment with the Cephea System.
You will have follow up exams at the following intervals.
• 30 days • 2 years • 3 months • 3 years • 6 months • 4 years • 1 year • 5 years
MAT-2511917 v1.0 | Item approved for U.S. use only.
*CAUTION: investigational device. Limited by federal (U.S.) Law to investigational use only.