ASD Closure
ATRIAL SEPTAL DEFECT
TREATMENT OPTIONS
An atrial septal defect (hole in heart) is a type of congenital heart defect that can be treated with catheter-based procedures to close the opening, known as ASD closure.
Other treatment options include open-heart surgery or medication that may be used in treating symptoms associated with the ASD.
DISCUSS ALL TREATMENT OPTIONS WITH YOUR DOCTOR
Your doctor can describe the risks and benefits and help you decide which option is right for you.
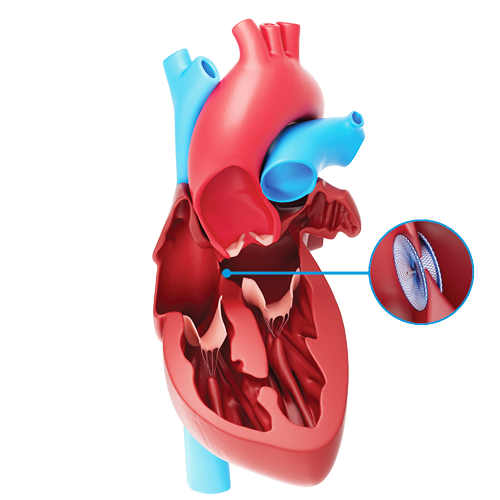
The Amplatzer™ Septal Occluder and
Amplatzer™ Multifenestrated Septal Occluder – "Cribriform"
The Amplatzer Septal Occluder is a device specifically designed to close an ASD. The Amplatzer Cribriform Occluder is a device specifically designed to close a multifenestrated ASD, a type of ASD consisting of many small holes rather than just one. Your doctor will choose the appropriate device for your specific ASD. The selected device is implanted during a catheter-based procedure and remains permanently implanted
Both devices are made from a braided nitinol, a metal with shape memory characteristics. This means the device will go back to its original shape even after it is stretched to pass through a catheter. The shapes of the Amplatzer Septal Occluder and Amplatzer Cribriform Occluder were specifically designed to seal ASDs and multifenestrated ASDs, respectively.

How the Amplatzer Septal Occluder closed a hole in Christy’s heart.
Despite being in good health, Abbott patient Christy had a stroke due to a hole in her heart. Christy shares how the Amplatzer Septal Occluder allowed her to get back on her feet with minimal downtime to continue training to run the Boston Marathon.
This testimonial relates an account of an individual’s response to the treatment. This patient’s account is genuine, typical, and documented. However, it does not provide any indication, guide, warranty, or guarantee as to the response other persons may have to the treatment. Responses to the treatment discusses can and do vary and are specific to the individual patient.
Frequently asked questions
- How do I know which treatment option is right for me?
Every person is unique. Your doctor is your best resource for learning about the treatment options available to you and the best course for your condition. Talk to your doctor and follow his or her advice for your care. Remember an ASD can result in unpleasant symptoms and increased health risk. With proper care, however, it can generally be managed with medication or closure. There are different advantages and complications for each approach, and you should discuss these with your doctors.
- What is involved with a catheter-based procedure?
A catheter-based procedure is a minimally invasive treatment option available to some people. The procedure involves making a small incision, typically in the groin, and inserting a small tube, called a catheter or sheath, to navigate through the blood vessels to the procedure site within the heart.
In persons with an ASD, the doctor guides the device through the catheter or sheath and deploys it in the ASD to seal the hole. Once the device is successfully placed defect, the doctor will carefully study its position using cardiac imaging systems. Once the physician is satisfied with the position, the device is released to remain permanently in the defect. The catheter or sheath is removed and the procedure is completed.
The procedure itself lasts about one to two hours and takes place in a heart catheterization laboratory, where many minimally invasive, non-surgical procedures are performed. Your doctor may give you an anesthetic, and you should not feel any significant discomfort.
- Who should not receive an Amplatzer Septal Occluder or Amplatzer Cribriform Occluder ?
If you have any of the following conditions you may not be a good candidate to receive the device.
- If you need to have surgery to fix other defects in your heart.
- If you have an infection anywhere in your body. You may receive the device only after the infection is gone.
- If you have a bleeding disorder, untreated ulcer, or if you are unable to take aspirin.
- If you are unable to take antiplatelet or anticoagulant therapy (i.e., blood thinning medications).
- If have blood clots in your heart.
- If you have a patent foramen ovale.
- If you, your heart, or your veins are very small or if there is any reason you cannot undergo the procedure.
- If the device would interfere with or come in contact with other structures in your heart.
- Can I travel with an implanted device? Will my device trigger airport security systems?
Your physician is your best resource for the answer to this question. Many patients find that with some extra planning and care they can enjoy traveling even with an implanted device. It is always wise to carry your patient ID card, just in case you encounter difficulties while traveling.
Though some people worry about airport security systems there is really no need for concern. The metal parts in Amplatzer occlusion devices are very small and usually do not trigger metal detector alarms. However, the sensitivity setting of the metal detector and other factors may affect how the metal detector responds to your device. Simply show your patient identification card to security personnel.
- What is a patient identification card? Will I need to carry it with me?
As a recipient of an implanted medical device, it is important to carry a patient identification card with you to identify yourself as having an implanted device. The patient ID card includes your name, implant date, your doctor’s contact information, and information about your device. You will be provided with this card after the procedure.
- Can I have this procedure if I am pregnant? What if I am a nursing mother?
The risk of increased x-ray exposure must be weighed against the potential benefits of this device. Your physician will ensure that care will be taken to minimize the radiation exposure to the fetus and mother.
It is unknown if the device affects breast milk. You should discuss this issue with your doctor.
- Will medical equipment interfere with my device?
Although most medical equipment will have no effect on your device, it is best to tell hospital personnel that you have an implanted device before you undergo any medical procedure. Magnetic resonance imaging (MRI) scans are generally acceptable, and your Amplatzer occlusion device has no known hazards when using a 3-tesla MRI. If an MRI is needed, simply inform the MRI staff about your implant.
- After the procedure, what symptoms should I seek medical help for?
If you experience any of following symptoms after the procedure: chest pain, numbness, sudden weakness, dizziness or rapid heartbeat, seek medical help immediately. An echocardiogram (ultrasound of the heart) should be performed.
- What risks are associated with the Amplatzer Septal Occluder or Amplatzer Cribriform Occluder?
There are certain potential risks associated with catheter-based procedures as well as additional risks that may be associated with the device. Your doctor is the best source of information about the risks of having an implanted device. Be sure to talk about all your questions and concerns.
Potential risks include, but are not limited:
Adverse events associated with the Amplatzer Septal Occluder
- Air embolus
- Allergic dye reaction
- Anesthesia reactions
- Apnea
- Arrhythmia
- Cardiac tamponade
- Death
- Embolization
- Fever
- Hypertension/hypotension
- Infection including endocarditis
- Need for surgery
- Pericardial effusion
- Perforation of vessel or mycardium
- Pseudoaneurysm including blood loss requiring transfusion
- Pleural effusion (excess fluid between the layers of tissue that line the lungs and chest activity)
- Tissue erosion
- Thrombus formation on discs
- Stroke
- Valvular regurgitation
Adverse events associated with the Amplatzer Cribriform Occluder
- Air embolus
- Allergic dye reaction
- Anesthesia reaction
- Apnea
- Arrhythmia
- Brachial plexus injury
- Cardiac perforation
- Death
- Device collapse due to structural failure
- Device embolization
- Device removal (due to embolization or misplacement)
- Erosion
- Fever
- Headache/migraines
- Hematoma/pseudoaneurysm including blood loss requiring transfusion
- Hyper; hypotension
- Infection including endocarditis
- Infectious endocarditis
- Pericardial effusion
- Perforation of vessel or myocardium
- Phrenic nerve injury
- Stroke/Transient ischemic attack
- Thrombus formation on the device surface with the risk of subsequent embolization
- Valvular regurgitation
- Vascular access site complications
You should also be aware that:
- People who are allergic to nickel may have an allergic reaction to this device.
- Some people have experienced tissue erosion, a very serious or life-threatening condition caused by the device rubbing against the wall of the heart and creating a hole. This may cause blood to build up in the sac that surrounds the heart. If too much blood builds up in this sac the heart will not be able to work properly. Symptoms of this may be shortness of breath and/or chest pain, fainting, and irregular heartbeat. If you have any of these symptoms, immediately call your doctor or go to the emergency room for an echocardiogram (ultrasound of the heart). Your doctor will be able to tell if you have this complication by doing this examination.
- As of May 2012, it is estimated that worldwide, erosion may occur in one to three of every 1000 patients. This means that the risk of you experiencing an erosion is estimated to be somewhere between 0.1% to 0.3% in Amplatzer™ Septal Occluder recipients and <1 in 10,000 in Amplatzer™Multifenestrated Septal Occluder recipients.
- The majority, almost 90%, of erosions occur within one year of being implanted, but some erosions have happened several years after implant.1
The information provided is not intended for medical diagnosis or treatment or as a substitute for professional advice. Consult with a physician or qualified healthcare provider for appropriate medical advice.
MAT-2305395 v3.0 | Item approved for U.S. use only.
