VSD Closure
VENTRICULAR SEPTAL DEFECT
TREATMENT OPTIONS
An ventricular septal defect is a hole in the two lower chambers of the heart and is a type of congenital heart defect. There are a number of treatment options for a VSD, and there is no single option that is right for every patient. You should talk with your doctor to learn about the best treatment option for you or your child; however, there are a few standard approaches of which you should be aware. One option is medication which may be appropriate to help in treating symptoms associated with the VSD.
Other treatment options include open-heart surgery and catheter-based procedures to close the hole in the heart. VSDs have been known to close spontaneously or become insignificant in size during the first one or two years of life.1 Therefore, your physician may recommend waiting a year or two to see if closure is necessary.
DISCUSS ALL TREATMENT OPTIONS WITH YOUR DOCTOR
Your doctor can describe the risks and benefits and help you decide which option is right for you.
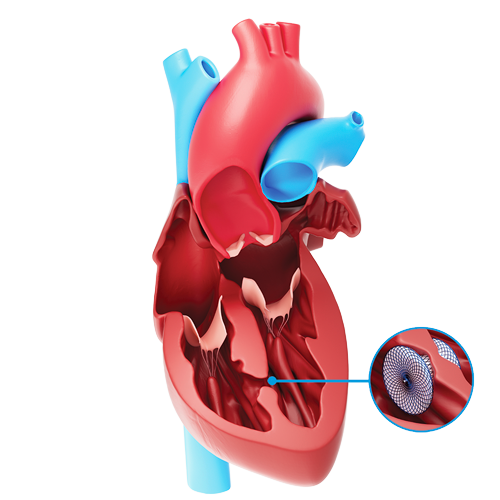
The Amplatzer™ VSD Occluders
The Amplatzer VSD Occluders are devices specifically designed to non-surgically close a VSD. The devices are placed in the VSD during catheter-based procedures and will remain permanently implanted.
The Amplatzer VSD Occluders are made from braided nitinol wires. Nitinol is a metal with shape memory characteristics, meaning the device will return to its original “memorized” shape even after it is stretched to pass through a catheter. The shape of the device was specifically designed to stop blood flow through a VSD.


See how patients like Jozef benefit from VSD closure
After receiving the Amplatzer™ VSD Occluder, Jozef was amazed at his energy and ability to walk long distances again. “I feel joy deep inside me—inside my heart.”
Frequently asked questions
- How do I know which treatment option is right for me?
Every person is unique. Your doctor is your best resource for learning about the treatment options available to you and the best course for your condition. Talk to your doctor and follow his or her advice for your care. Keep in mind that a VSD can result in unpleasant symptoms and increased health risk. With proper care, however, it can generally be managed with medication or closure.
- What is involved with a catheter-based procedure?
A catheter-based procedure is a minimally invasive treatment option available to some people. The procedure involves making a small incision, typically in the groin, and inserting a small tube, called a catheter or sheath, to navigate through the blood vessels to the procedure site within the heart. In people with a VSD, the doctor will then guide the device through the catheter or sheath to reach the VSD. Once the device is placed in the hole, the doctor will carefully study its position using cardiac imaging systems. Once the physician is satisfied with the position, the device is released to remain permanently in the hole. The catheter or sheath is removed and the procedure is completed.
The procedure itself should last about one to two hours and will take place in a heart catheterization laboratory, where many minimally invasive, non-surgical procedures are performed. Your doctor may give you an anesthetic, and you should not feel any significant discomfort.
- Who should not receive an Amplatzer VSD Occluder?
If you have any of the following conditions you may not be a good candidate to receive the Amplatzer VSD Occluder.
- If your VSD is too close to your heart’s valves
- If your VSD is a perimembranous VSD
- If your VSD resulted from a heart attack
- If you weigh less than 5.2 kg (11.4 lbs)
- If you have an infection anywhere in your body, you may receive the device only after the infection is gone
- If you are unable to take antiplatelet medication
- What is a patient identification card? Will I need to carry it with me?
As a device recipient, it is important to carry a patient identification card with you to identify yourself as having an implanted device. The patient ID card includes your name, implant date, your doctor’s contact information and information about your device. You will be provided with this card after the procedure.
- Can I travel with an implanted device? Will my device trigger airport security systems?
Your physician is your best resource for the answer to this question. Many people find that with some extra planning and care they can enjoy traveling even with an implanted device. It is always wise to carry your patient ID card, just in case you encounter difficulties while traveling.
Though some people worry about airport security systems there is really no need for concern. The metal parts in Amplatzer occlusion devices are very small and usually do not trigger metal detector alarms. However, the sensitivity setting of the metal detector and other factors may affect how the metal detector responds to your device. Simply show your patient identification card to security personnel.
- Will medical equipment interfere with my device?
Although most medical equipment will have no effect on your device, it is best to tell hospital personnel that you have an implanted device before you undergo any medical procedure. Magnetic resonance imaging (MRI) scans are generally acceptable, and your Amplatzer occlusion device has no known hazards when using a 3-tesla MRI. If an MRI is needed, simply inform the MRI staff about your implant.
- Can I have this procedure if I am pregnant? What if I am a nursing mother?
The risk of increased x-ray exposure must be weighed against the potential benefits of this device. Your physician will ensure that care will be taken to minimize the radiation exposure to the fetus and mother.
It is unknown if the device affects breast milk. You should discuss this issue with your doctor.
- What happens after the procedure?
Because the procedure is minimally invasive, the recovery will likely be quick and easy. Many people are discharged from the hospital within 24 hours. Your doctor can provide guidelines for activities and medications. He or she may prescribe drugs to be taken at home to continue treatment and recovery. The decision to prescribe these is at the discretion of your doctor. Many doctors require follow-up appointments over the next year to ensure the recovery is going well. What to expect during and after the procedure will vary. Discuss all questions and concerns you have with your doctor.
- After the procedure, what symptoms should I seek medical help for?
If you experience any of following symptoms after the procedure: chest pain, numbness, sudden weakness, dizziness or rapid heartbeat, seek medical help immediately. An echocardiogram (ultrasound of the heart) should be performed.
- How long will it take me to recover, what activities should be avoided after the procedure, and when can they resume?
Every person recovers differently, and your doctor can help determine when activities can be resumed. In general all strenuous activity should be avoided for one month after the procedure.
- Will I be able to feel the device?
No, you will not be able to feel the device once it’s implanted.
- What risks are associated with the Amplatzer VSD Occluder?
There are certain potential risks associated with catheter-based procedures as well as additional risks that may be associated with the device. Your doctor is the best source of information about the risks of having an implanted device. Be sure to talk about all your questions and concerns.
Potential risks include, but are not limited to:
- Air embolus
- Allergic drug reaction
- Allergic dye reaction
- Anemia (a decrease in or lesser than normal quantity of healthy red blood cells)
- Anesthesia reaction
- Apnea (temporary absence of breathing)
- Arrhythmia (loss of regular heart rhythm)
- Arterial pulse loss (decreased amount of blood flow through an artery)
- Atelectasis (collapse of part or all of the lung causing lack of gas exchange within alveoli)
- Bacterial endocarditis (infection that causes swelling of the lining of the heart and its valves)
- Blood loss requiring transfusion
- Brachial plexus injury (injury to the nerves in the arm or lower neck)
- Cardiac arrest (unexpected loss of heart function)
- Cardiomyopathy (deterioration of the function of the heart muscle)
- Chest pain
- Cyanosis (bluish discoloration of the skin due to lack of oxygen)
- Death
- Device embolization (dislodging of the device)
- Device fracture
- Fever
- Headache/Migraine
- Heart block (an interruption in the normal rhythm of the heart beat)
- Hypotension (abnormally high/low blood pressure)
- Myocardial infarction (heart attack)
- Perforation of vessel or myocardium (piercing of a vessel or the heart)
- Peripheral embolism (when a small clot or piece of debris passes through the peripheral system causing decreased or blocked blood flow in an artery or vein)
- Stridor (high pitched wheezing)
- Stroke
- Subaortic stenosis (abnormal narrowing of the left ventricle below the aorta)
- Thrombus formation on device (blood clot)
- Vascular access site injury
- Venous thrombosis (condition resulting in blood clots forming in a vein)
- Vomiting
You should also be aware that patients allergic to nickel may have an allergic reaction to this device.
The information provided is not intended for medical diagnosis or treatment or as a substitute for professional advice. Consult with a physician or qualified healthcare provider for appropriate medical advice.
MAT-2305396 v4.0 | Item approved for U.S. use only.