PDA Closure in
Premature Infants
WHAT IS THE AMPLATZER PICCOLO™ OCCLUDER AND HOW DOES IT
TREAT PDAS IN PREMATURE INFANTS
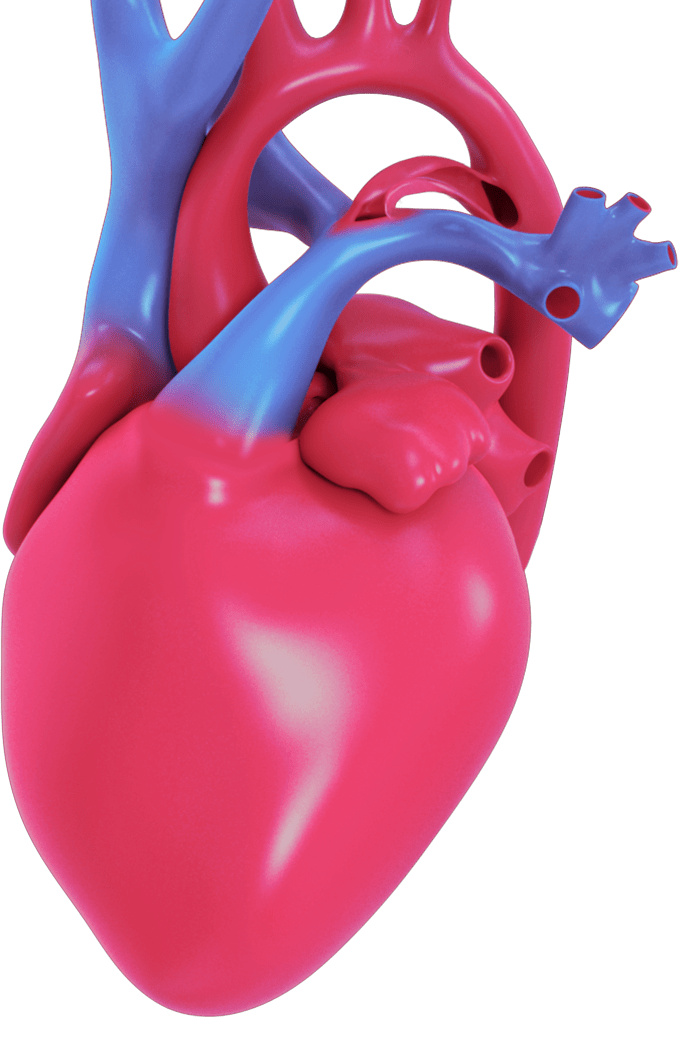
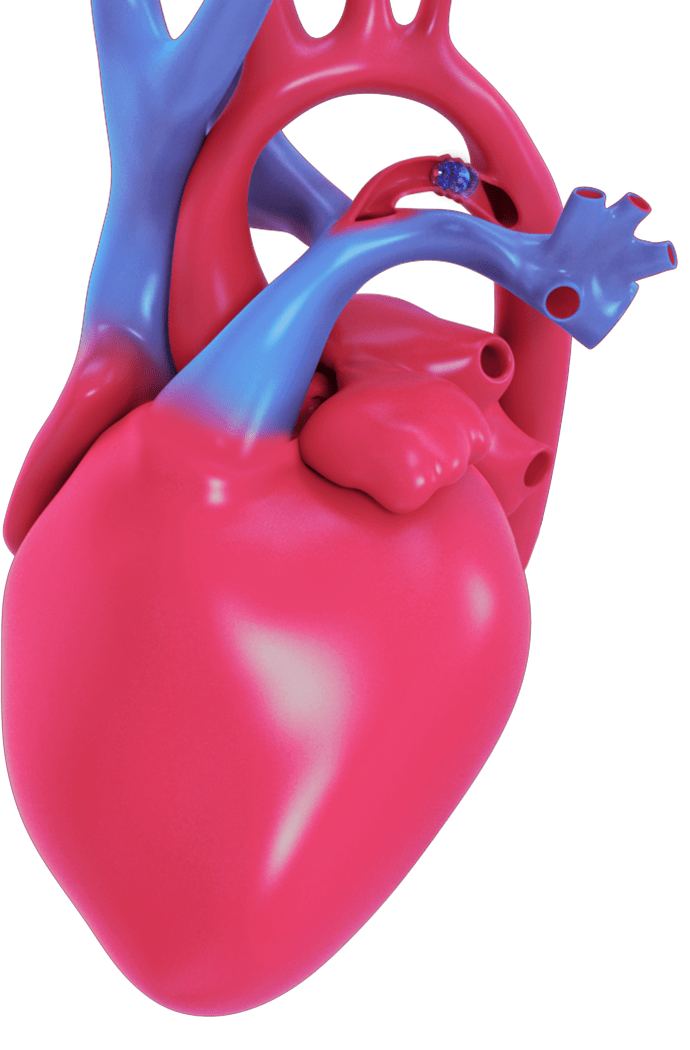
The Amplatzer Piccolo™ Occluder is the only minimally invasive PDA closure device that is FDA approved for premature infants.
The device can be safely implanted to help seal the opening in the heart (close the PDA) without open heart surgery and your baby's heart tissue will eventually grow over the device, sealing the hole. Early PDA closure can lead to improved outcomes for your child.

DISCUSS ALL TREATMENT OPTIONS WITH YOUR DOCTOR
Your doctor can describe the risks and benefits and help you decide which option is right for your child.
Patient Guide for Nonsurgical Closure of a Patent Ductus Arteriosus (PDA)
Download the PDA parent guide to learn more about non-surgical closure of the PDA in premature infants with the Amplatzer Piccolo Occluder.

THE PICCOLO DEVICE SAVED
LITTLE TONY’S LIFE BY CLOSING A PDA
“Because he was able to have this procedure, that time in the NICU is a distant memory” – Little Tony’s Dad
Abbott patient, Little Tony, was born prematurely at 26 weeks and weighed only two pounds. He had a patent ductus arteriosus (PDA), a hole in his heart that had failed to close.
Tony’s parents share his PDA diagnosis and how being the first premature baby to receive the Amplatzer Piccolo Occluder changed his life forever within a matter of hours.
This testimonial relates an account of an individual’s response to the treatment. This patient’s account is genuine, typical, and documented. However, it does not provide any indication, guide, warranty, or guarantee as to the response other persons may have to the treatment. Responses to the treatment discusses can and do vary and are specific to the individual patient.
You're not alone. Every year, nearly 12,000 premature babies in the U.S., like Tony, are born with an open PDA, requiring urgent treatment to survive.
FREQUENTLY ASKED QUESTIONS
- How is PDA treated?
There are multiple treatment options for a PDA, and you should talk with your child’s doctor to learn about the best treatment option for them. Medications are usually given as a first treatment option, which may help close the PDA or treat symptoms of the PDA. Occasionally a minimally invasive procedure or even surgery may be needed.1
- Which PDA treatment option is right for my child?
Every infant is unique. Your child’s doctor is your best resource for learning about the PDA treatment options available to your child and the best course for their condition. Talk to your child’s doctor and follow his or her advice for their care. Remember, a PDA can result in life threatening symptoms and increased health risk. With proper care, however, it can generally be managed with medication or PDA closure.
- Who should not receive the Amplatzer Piccolo Occluder?
If your child has any of the following conditions, they may not be a good candidate to receive the Amplatzer Piccolo Occluder.
- If your child weighs less than 700 grams at the time of the procedure
- If your child is fewer than 3 days old at the time of the procedure
- If your child has a narrowing in the aorta or in the left pulmonary artery
- If your child has high blood pressure in the pulmonary arteries and the blood flow to the rest of the body must go through the PDA
- If your child's PDA length is shorter than 3mm
- If your child's PDA diameter at the narrowest portion is greater than 4mm
- If your child has an active infection
- If your child has a clot (thrombus) in their heart that may interfere with the implant procedure
- What is involved in the procedure?
Closing a PDA with the Amplatzer Piccolo Occluder is a minimally invasive procedure that does not require open heart surgery.
The procedure usually takes 1-2 hours and is performed by a physician with specialized training.
- What happens after the procedure?
Additional monitoring will continue in the NICU to address the other medical needs of your child, however the closing of the hole in your child's heart may have a positive effect on some of the other medical issues your child may be experiencing. After your child is discharged from the NICU, your doctor will schedule follow-up appointments to ensure your child's recovery is continuing to progress.
- How long will it take my child to recover?
Your child will remain in NICU as doctors address any other medical issues with your child, however recovery from device closure can be almost immediate. Every infant recovers differently, and your doctor can share the individualized plan for your child.
- Is the device painful, or will my child be able to feel the device?
Once the procedure is over, your child will not feel the device, or pain because of the device.
- What risks are associated with the Amplatzer Piccolo Occluder?
There are certain potential risks associated with catheter-based procedures as well as additional risks that may be associated with the device. Your child’s doctor is the best source of information about the risks of having an implanted device. Be sure to talk about all your questions and concerns.
Potential risks include, but are not limited to:
- Air embolus
- Allergic reaction
- Anemia
- Anesthesia reactions
- Apnea
- Arrhythmia
- Bleeding
- Cardiac perforation
- Cardiac tamponade
- Chest pain
- Device embolization
- Device erosion
- Death
- Endocarditis
- Fever
- Headache/migraine
- Hemolysis
- Hematoma
- Hypertension
- Hypotension
- Infection
- Myocardial infarction
- Palpitations
- Partial obstruction of aorta
- Partial obstruction of pulmonary artery
- Pericardial effusion
- Pericarditis
- Peripheral embolism
- Pleural effusion
- Pulmonary embolism
- Re-intervention for device removal
- Respiratory distress
- Stroke
- Thrombus
- Transient ischemic attack
- Valvular regurgitation
- Vascular access site injury
- Vascular Occlusion
- Vessel perforation
The information provided is not intended for medical diagnosis or treatment or as a substitute for professional advice. Consult with a physician or qualified healthcare provider for appropriate medical advice.
MAT-2305647 v3.0 | Item approved for U.S. use only.
